Chemistry:1,2-Dimorpholinoethane
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H20N2O2 | |
| Molar mass | 200.282 g·mol−1 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H315, H317, H318, H319, H335 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P271, P272, P280, P302+352, P304+340, P305+351+338, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P317Script error: No such module "Preview warning".Category:GHS errors, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P332+317Script error: No such module "Preview warning".Category:GHS errors, P333+313, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
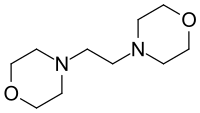
1,2-Dimorpholinoethane is an organic chemical and specifically a nitrogen-oxygen heterocyclic compound. At room temperature it is a solid with a melting point of 75 °C.[2] It has two tertiary amines in the same molecule meaning it is ideal for use as a polyurethane catalyst.[3] It has the CAS Registry Number 1723-94-0 and is TSCA and REACH registered and on EINECS with the number 217-026-5.[4] The IUPAC name is 4-(2-morpholin-4-ylethyl)morpholine and the chemical formula C10H20N2O2.[5]
Synonyms
Section reference.[6]
- 4,4'-Ethylenedimorpholine
- 4,4'-(Ethane-1,2-diyl)bismorpholine
- Morpholine, 4,4'-(1,2-ethanediyl)bis-
- 1,2-Di-N-morpholinylethane
- Morpholine,4,4'-(1,2-ethanediyl)bis-
- Morpholine, 4,4'-ethylenedi-
Uses and synthesis
As the molecule has two tertiary nitrogen atoms in the molecule, the substance finds use as a catalyst for polyurethane[7] including PU foams.[8][9][10][11][12]
1,2-Dimorpholinoethane has been used to make transition metal complexes. As there are two nitrogen atoms in the molecule it acts as a bidentate ligand in these complexes.[13] These complexes have then be used in antibacterial applications.[14]
Toxicity
The toxicity of the compound and tertiary amines in general has been studied and published.[15]
References
- ↑ "1,2-Dimorpholinoethane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/72888#section=Safety-and-Hazards.
- ↑ "CAS Common Chemistry". https://commonchemistry.cas.org/detail?cas_rn=1723-94-0.
- ↑ "1,2-Dimorpholinoethane | C10H20N2O2 | ChemSpider". http://www.chemspider.com/Chemical-Structure.65720.html.
- ↑ "Substance Information - ECHA" (in en-GB). https://echa.europa.eu/substance-information/-/substanceinfo/100.015.478.
- ↑ "4,4'-(ethane-1,2-diyl)bismorpholine CAS#: 1723-94-0" (in en). https://www.chemicalbook.com/ProductChemicalPropertiesCB8925275_EN.htm.
- ↑ PubChem. "1,2-Dimorpholinoethane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/72888.
- ↑ Van Maris, Roger; Tamano, Yutaka; Yoshimura, Hiroyuki; Gay, Kenneth M. (July 2005). "Polyurethane Catalysis by Tertiary Amines" (in en). Journal of Cellular Plastics 41 (4): 305–322. doi:10.1177/0021955X05055113. ISSN 0021-955X. http://journals.sagepub.com/doi/10.1177/0021955X05055113.
- ↑ Bacaloglu, R.; Cotarcâ, L.; Marcu, N.; Tölgyi, St (1988). "Kinetics and mechanism of isocyanate reactions. II. Reactions of Aryl Isocyanates with Alcohols in the presence ob tertiary amines" (in en). Journal für Praktische Chemie 330 (4): 530–540. doi:10.1002/prac.19883300406. https://onlinelibrary.wiley.com/doi/10.1002/prac.19883300406.
- ↑ Adam, Norbert; Avar, Geza; Blankenheim, Herbert; Friederichs, Wolfgang; Giersig, Manfred; Weigand, Eckehard; Halfmann, Michael; Wittbecker, Friedrich-Wilhelm et al. (2005). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_665.pub2.
- ↑ "Jeffcat Amine Catalysts for the Polyurethane Industry". 2006. http://www.huntsman.com/performance_products/Media/JEFFCAT_Catalyst_Trifold_bulletin.pdf.
- ↑ Muuronen, Mikko; Deglmann, Peter; Tomović, Željko (2019-06-21). "Design Principles for Rational Polyurethane Catalyst Development" (in en). The Journal of Organic Chemistry 84 (12): 8202–8209. doi:10.1021/acs.joc.9b01319. ISSN 0022-3263. PMID 31125228. https://pubs.acs.org/doi/10.1021/acs.joc.9b01319.
- ↑ Marsella, John A. (February 1987). "Homogeneously catalyzed synthesis of .beta.-amino alcohols and vicinal diamines from ethylene glycol and 1,2-propanediol" (in en). The Journal of Organic Chemistry 52 (3): 467–468. doi:10.1021/jo00379a035. ISSN 0022-3263. https://pubs.acs.org/doi/abs/10.1021/jo00379a035.
- ↑ Mazhar-Ul-Haque; Sakhawat Hussain, M.; Ahmed, Jamil (1992-02-01). "Tetrahedral complexes of 1,2-dimorpholinoethane and 1,3-dimorpholinopropane: Crystal and molecular structure of dichloro(1,2-dimorpholinoethane)cobalt(II)" (in en). Journal of Crystallographic and Spectroscopic Research 22 (1): 37–41. doi:10.1007/BF01161361. ISSN 1572-8854. https://doi.org/10.1007/BF01161361.
- ↑ Goudarziafshar, Hamid; Rezaeivala, Majid; Khosravi, Fayezeh; Abbasityula, Yunes; Yousefi, Somaieh; Özbek, Neslihan; Eigner, Václav; Dušek, Michal (2015-01-01). "Synthesis, characterization and crystal structures of new Zinc(II) and Nickel(II) complexes containing morpholine moiety and their antibacterial studies" (in en). Journal of the Iranian Chemical Society 12 (1): 113–119. doi:10.1007/s13738-014-0462-2. ISSN 1735-2428. https://doi.org/10.1007/s13738-014-0462-2.
- ↑ Albrecht, William N; Stephenson, Richard L (1988). "Health hazards of tertiary amine catalysts". Scandinavian Journal of Work, Environment & Health 14 (4): 209–219. doi:10.5271/sjweh.1930. ISSN 0355-3140. PMID 3051334.
 |

