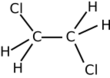
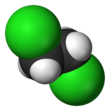
Chemistry:1,2-Dichloroethane
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Dichloroethane | |||
| Other names
Ethylene dichloride
Ethylene chloride 1,2-DCA 1,2-DCE DCE[1] Ethane dichloride Dutch liquid, Dutch oil Freon 150 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 605264 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| EC Number |
| ||
| 49272 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| C2H4Cl2 | |||
| Molar mass | 98.95 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | characteristic, pleasant chloroform-like odor[2] | ||
| Density | 1.253 g/cm3, liquid | ||
| Melting point | −35 °C (−31 °F; 238 K) | ||
| Boiling point | 84 °C (183 °F; 357 K) | ||
| 0.87 g/100 mL (20 °C) | |||
| Viscosity | 0.84 mPa·s at 20 °C | ||
| Structure | |||
| 1.80 D | |||
| Hazards | |||
| Main hazards | Toxic, flammable, carcinogenic | ||
| GHS pictograms |   
| ||
| GHS Signal word | Danger | ||
| H225, H302, H315, H319, H335, H350 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P281, P301+312, P302+352, P303+361+353, P304+340, P305+351+338, P308+313, P312, P321, P330, P332+313, P337+313 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 13 °C (55 °F; 286 K) | ||
| Explosive limits | 6.2–16%[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
3000 ppm (guinea pig, 7 h) 1000 ppm (rat, 7 h)[3] | ||
LCLo (lowest published)
|
1217 ppm (mouse, 2 h) 1000 ppm (rat, 4 h) 3000 ppm (rabbit, 7 h)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 50 ppm C 100 ppm 200 ppm [5-minute maximum peak in any 3 hours][2] | ||
REL (Recommended)
|
Ca TWA 1 ppm (4 mg/m3) ST 2 ppm (8 mg/m3)[2] | ||
IDLH (Immediate danger)
|
Ca [50 ppm][2] | ||
| Related compounds | |||
Related haloalkanes
|
Methyl chloride Methylene chloride 1,1,1-Trichloroethane | ||
Related compounds
|
Ethylene Chlorine Vinyl chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts.[4] 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water (at a boiling point of 70.5 °C or 158.9 °F or 343.6 K) and other chlorocarbons.[5]
History
In 1794, physician Jan Rudolph Deiman, merchant Adriaan Paets van Troostwijk, chemist Anthoni Lauwerenburg, and botanist Nicolaas Bondt, under the name of Society of Dutch Chemists (Dutch: Gezelschap der Hollandsche Scheikundigen), were the first to produce 1,2-dichloroethane from olefiant gas (oil-making gas, ethylene) and chlorine gas.[6] Although the Gezelschap in practice did not do much in-depth scientific research, they and their publications were highly regarded. Part of that acknowledgement is that 1,2-dichloroethane was called "Dutch oil" in old chemistry. This is also the origin of the archaic term "olefiant gas" (oil-making gas) for ethylene, for in this reaction it is ethylene that makes the Dutch oil. And "olefiant gas" is the etymological origin of the modern term "olefins", the family of hydrocarbons of which ethylene is the first member.
Production
Nearly 20 million tons of 1,2-dichloroethane are produced annually in the United States , Western Europe, and Japan .[7] Production is primarily achieved through the iron(III) chloride-catalysed reaction of ethylene and chlorine:
- H
2C=CH
2 (g) + Cl
2 (g) → ClC
2H
4Cl (l) (ΔH⊖r = −218 kJ/mol)
1,2-dichloroethane is also generated by the copper(II) chloride-catalysed oxychlorination of ethylene:
- H
2C=CH
2 + 2 HCl + 1/2 O
2 → ClC
2H
4Cl + H
2O
Uses
Vinyl chloride monomer (VCM) production
Approximately 95% of the world's production of 1,2-dichloroethane is used in the production of vinyl chloride monomer (VCM, chloroethene) with hydrogen chloride as a byproduct. VCM is the precursor to polyvinyl chloride.
- ClC
2H
4Cl → H
2C=CHCl + HCl
The hydrogen chloride can be re-used in the production of more 1,2-dichloroethane via the oxychlorination route described above.[8]
Other uses
1,2-Dichloroethane has been used as degreaser and paint remover but is now banned from use due to its toxicity and possible carcinogenity. As a useful 'building block' reagent, it is used as an intermediate in the production of diverse organic compounds such as ethylenediamine and higher ethyleneamines.[9] In the laboratory it is occasionally used as a source of chlorine, with elimination of ethene and chloride.
Via several steps, 1,2-dichloroethane is a precursor to 1,1,1-trichloroethane. Historically, before leaded petrol was phased out, 1,2-dichloroethane was used as an anti-knock additive in petrol as a scavenging agent to prevent lead buildup.[10]
Safety
1,2-Dichloroethane is highly flammable[11] and releases hydrochloric acid when combusted:
- ClC
2H
4Cl + 5/2 O
2 → 2 CO
2 + H
2O + 2 HCl
It is also toxic (especially by inhalation due to its high vapour pressure) and possibly carcinogenic. Its high solubility and 50-year half-life in anoxic aquifers make it a perennial pollutant and health risk that is very expensive to treat conventionally, requiring a method of bioremediation.[12] While the chemical is not used in consumer products manufactured in the U.S., a case was reported in 2009 of molded plastic consumer products (toys and holiday decorations) from China that released 1,2-dichloroethane into homes at levels high enough to produce cancer risk.[13][14] Substitutes are recommended and will vary according to application. Dioxolane and toluene are possible substitutes as solvents. Dichloroethane is unstable in the presence of aluminium metal and, when moist, with zinc and iron.[citation needed]
References
- ↑ Staff writer. "Standard Abbreviations and Acronyms". http://pubs.acs.org/paragonplus/submission/joceah/joceah_abbreviations.pdf. "DCE: 1,2-dichloroethane"
- ↑ 2.0 2.1 2.2 2.3 2.4 NIOSH Pocket Guide to Chemical Hazards. "#0271". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0271.html.
- ↑ 3.0 3.1 "Ethylene dichloride". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/107062.html.
- ↑ "Toxic Substances – 1,2-Dichloroethane". https://wwwn.cdc.gov/TSP/index.aspx?toxid=110.
- ↑ Manfred Rossberg, Wilhelm Lendle, Gerhard Pfleiderer, Adolf Tögel, Eberhard-Ludwig Dreher, Ernst Langer, Heinz Rassaerts, Peter Kleinschmidt, Heinz Strack, Richard Cook, Uwe Beck, Karl-August Lipper, Theodore R. Torkelson, Eckhard Löser, Klaus K. Beutel, Trevor Mann "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry. 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2.
- ↑ Deimann, van Troostwyk, Bondt and Louwrenburgh (1795) "Ueber die Gasarten, welche man aus Verbindungen von starker Vitriolsäure und Alkohol erhält" (On the types of gases which one obtains from combinations of strong vitriolic acid and alcohol), Chemische Annalen ... , 2 : 195-205, 310-316, 430-440. The production and characterization of 1,2-dichloroethane appear on pages 200-202. The investigators were trying to detect the presence of carbon (Kohl) in ethylene (Luft, literally, "air") by adding chlorine (zündend Salzgas, literally, "burning gas from salt"). Instead of the expected soot, an oil (Oehl) formed.
- ↑ J.A. Field; R. Sierra-Alvarez (2004). "Biodegradability of chlorinated solvents and related chlorinated aliphatic compounds". Rev. Environ. Sci. Biotechnol. 3 (3): 185–254. doi:10.1007/s11157-004-4733-8.
- ↑ "Ethylene Dichloride – Chemical Economics Handbook (CEH) – IHS Markit". https://www.ihs.com/products/ethylene-dichloride-chemical-economics-handbook.html.
- ↑ Srivasan Sridhar.; Richard G. Carter (2001), "Diamines and Higher Amines, Aliphatic", Kirk-Othmer Encyclopedia of Chemical Technology, New York: John Wiley, doi:10.1002/0471238961.0409011303011820.a01.pub2, ISBN 9780471238966, http://onlinelibrary.wiley.com/book/10.1002/0471238961
- ↑ Seyferth, D. (2003). "The Rise and Fall of Tetraethyllead. 2". Organometallics 22 (25): 5154–5178. doi:10.1021/om030621b.
- ↑ "1,2-Dichoroethane MSDS." Mallinckrodt Chemicals. 19 May 2008. Web. <http://hazard.com/msds/mf/baker/baker/files/d2440.htm>.
- ↑ S. De Wildeman; W. Verstraete (25 March 2003). "The quest for microbial reductive dechlorination of C2 to C4 chloroalkanes is warranted". Appl. Microbiol. Biotechnol. 61 (2): 94–102. doi:10.1007/s00253-002-1174-6. PMID 12655450.
- ↑ "Toxic Christmas: Plastic Ornaments May Pollute Your Air". http://www.rodale.com/plastic-christmas-ornaments?page=0%2C0.
- ↑ Doucette, WJ; Hall, AJ; Gorder, KA (Winter 2010). "Emissions of 1, 2-Dichloroethane from Holiday Decorations as a Source of Indoor Air Contamination". Ground Water Monitoring & Remediation 30 (1): 67–73. doi:10.1111/j.1745-6592.2009.01267.x.
External links
- Gezelschap der Hollandsche Scheikundigen
- ChemicalLand compound database
- Environmental Chemistry compound database
- Merck Chemicals database
- National Pollutant Inventory – 1,2 Dichlorethane Fact Sheet
- Locating and estimating air emissions from sources of ethylene dichloride, EPA report EPA-450/4-84-007d, March 1984
 |