Chemistry:1,1,3,3-Tetramethyl-1,3-divinyldisiloxane
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,3,3-Tetramethyl-1,3-divinyldisiloxane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H18OSi2 | |
| Molar mass | 186.401 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.811 g/cm3 |
| Melting point | −99.7 °C (−147.5 °F; 173.5 K) |
| Boiling point | 139 °C (282 °F; 412 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H225, H226, H315, H319, H335, H413 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P403+235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
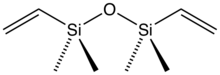
1,1,3,3-Tetramethyl-1,3-divinyldisiloxane (also referred to as tetramethyldivinyldisiloxane) is the organosilicon compound with the formula O(SiMe2CH=CH2)2.
Tetramethyldivinyldisiloxane is a colorless liquid that is employed as a ligand in organometallic chemistry and homogeneous catalysis.[1] The ligand is a component of Karstedt's catalyst. It was first prepared by hydrolysis of vinyldimethylmethoxysilane, (CH2=CH)Me2SiOMe.[2]
References
- ↑ Krause, Jochen; Cestaric, Günter; Haack, Karl-Josef; Seevogel, Klaus; Storm, Werner; Pörschke, Klaus-Richard (1999). "1,6-Diene Complexes of Palladium(0) and Platinum(0): Highly Reactive Sources for the Naked Metals and [L−M0] Fragments". Journal of the American Chemical Society 121 (42): 9807–9823. doi:10.1021/ja983939h.
- ↑ Kantor, Simon W.; Osthoff, Robert C.; Hurd, Dallas T. (1955). "Methylvinylpolysiloxanes". Journal of the American Chemical Society 77 (6): 1685–1687. doi:10.1021/ja01611a090.
 |

