Chemistry:Β-Naphthol methyl ether
From HandWiki
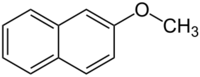
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methoxynaphthalene[1] | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H10O | |
| Molar mass | 158.200 g·mol−1 |
| Melting point | 73–75 °C (163–167 °F; 346–348 K) |
| Boiling point | 274 °C (525 °F; 547 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
β-Naphthol methyl ether, also called 2-methoxynaphthalene or yara yara,[2] is a stabilizer found in gunpowder, particularly smokeless gunpowders. It is soluble in alcohol, and insoluble in water and dipropylene glycol.
Studies have also been done on the antiinflammatory effect of β-naphthol methyl ether[3] and on how it behaves in time-resolved resonance Raman studies.[4]
Synthesis and uses
Nerolin can be prepared by alkylation of β-naphthol with dimethyl sulfate. It has a faint but persistent odor and used to be a scented compound found in soap and other products.[5]
References
- ↑ 1.0 1.1 1.2 "CHAPTER P-6. Applications to Specific Classes of Compounds". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 703. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ↑ "2-methoxynaphthalene", The Good Scents Company, 2012
- ↑ "Synthesis of 2-methoxynaphthalene derivatives as potential anti-inflammatory agents" (in it). Farmaco Sci 37 (3): 171–178. 1982. PMID 7067812.
- ↑ Mohapatra, Himansu; Umapathy, S. (2010). "Time-Resolved Resonance Raman Studies on Proton-Induced Electron-Transfer Reaction from Triplet Excited State of 2-Methoxynaphthalene to Decafluorobenzophenone". The Journal of Physical Chemistry A 114 (47): 12447–12451. doi:10.1021/jp109821r. PMID 21058635. Bibcode: 2010JPCA..11412447M.
- ↑ Mann, Frederick George (1952). Practical Organic Chemistry. Longmans, Green & Co. pp. 175.
 |

