Biology:Telomeres in the cell cycle
Telomeres, the caps on the ends of eukaryotic chromosomes, play critical roles in cellular aging and cancer. An important facet to how telomeres function in these roles is their involvement in cell cycle regulation.
Eukaryotic cells
Because eukaryotic chromosomes are linear and because DNA replication by DNA polymerase requires the presence of an RNA primer that is later degraded, eukaryotic cells face the end-replication problem.[1] This problem makes eukaryotic cells unable to copy the last few bases on the 3’ end of the template DNA strand, leading to chromosome—and, therefore, telomere—shortening every S phase.[2] Measurements of telomere lengths across cell types at various ages suggest that this gradual chromosome shortening results in a gradual reduction in telomere length at a rate of approximately 25 nucleotides per year.[3]
Cell cycle enablers and regulators
The telomere-shelterin complexes that cap all eukaryotic chromosomes ensure that healthy cells can progress through the cell cycle by preventing the cellular DNA damage response from identifying chromosome ends as double-stranded breaks (DSBs).[4][5] Without a protective cap, chromosome ends would appear identical to intrachromosomal DSBs. These DSBs activate a DNA damage response pathway that halts the cell cycle until the breaks are repaired. This checkpoint pathway is initiated in S. cerevisiae by recruitment of protein kinases Mec1 and Tel1 and in mammals by recruitment of protein kinases ATR and ATM.[6] Regarding DSB repair, eukaryotes generally use two strategies: non-homologous end joining (NHEJ), which involves rapid reattachment of the broken ends; and homologous recombination (HR), which involves the use of a homologous DNA sequence to repair the break. Because HR requires a homologous sequence, its use is restricted to S/G2 phase.[7] (Interestingly, as with many other aspects of the cell cycle,[8] cyclin-dependent kinases are responsible for downregulating NHEJ during S/G2 phase to ensure use of the more accurate HR.[9]) As shown in Figure 1A, telomere-shelterin complexes contain motifs that inhibit the DNA damage checkpoint, NHEJ, and HR.
Initial work on the role of telomere-bound protein complexes in S. cerevisiae elucidated the mechanism by which these complexes prevent checkpoint activation and DSB repair of chromosome ends. The two major protein complexes that bind to telomeric DNA in S. cerevisiae are:
- the Cdc13-Stn1-Ten1 (CST) complex, which binds the single-stranded DNA (ssDNA) of the 3’ G-rich overhang at the end of the telomere, and
- the Rif1-Rif2-Rap1 complex, which binds the double-stranded DNA (dsDNA) preceding the 3’ overhang.
The CST complex and Rif1 prevent Mec1 recruitment, thereby preventing checkpoint activation.[5][10] Meanwhile, Rif2 and Rap1 inhibit NHEJ: knocking out Rif2 or Rap1 results in longer telomeres as measured by PCR, indicating that NHEJ occurred.[11] These knockout strains (unlike strains lacking functional CST or Rif1) continue to cycle, further suggesting that Rif2 and Rap1 are not involved in inhibiting checkpoint activation.[12]
Analogously, proteins that bind to human telomeres as part of the shelterin complex enable cell cycle progress and prevent erroneous DSB repair. POT1 protein binds to ssDNA, prevents checkpoint activation through inhibiting ATR recruitment, and prevents HR;[13][14] RAP1, a GTPase, binds to dsDNA and prevents HR;[15] and TRF2 protein (also known as TERF2) binds to dsDNA, prevents checkpoint activation through inhibiting ATM recruitment, and prevents NHEJ.[16][17] TRF2 is unique among these proteins in its role in the formation and maintenance of T-loops: lariat structures formed by the folding of the ssDNA overhang back onto the dsDNA. T-loops may further inhibit the binding of checkpoint activation proteins.[18]
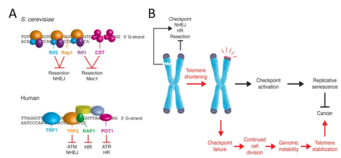
As telomeres shorten as a natural consequence of repeated cell division or due to other factors, such as oxidative stress,[19] shelterin proteins lose the ability to bind to telomeric DNA. When telomeres reach a critically short length, sufficient shelterin proteins to inhibit checkpoint activation are not available, although NHEJ and HR generally remain inhibited at this point.[5] This loss of inhibition is one reason why telomere shortening causes senescence (Figure 1B).
Telomeres and cell cycle deregulation
Almost all cancer cells have shortened telomeres.[20] This may seem counter-intuitive, as short telomeres should activate the ATR/ATM DNA damage checkpoint and thereby prevent division. Resolving the question of why cancer cells have short telomeres led to the development of a two-stage model for how cancer cells subvert telomeric regulation of the cell cycle. First, the DNA damage checkpoint must be inactivated to allow cells to continue dividing even when telomeres pass the critical length threshold. This requirement follows not only from the discussion above but also from in vivo evidence showing the function of this checkpoint in precancerous lesions and its dysfunction in late-stage tumors.[21] Second, to survive after disabling the DNA damage checkpoint, precancerous cells must activate mechanisms to extend their telomeres.[22][23] As a result of the continued division past the point of normal senescence, the telomeres of these cells become too short to prevent NHEJ(Non Homologous End Joining) and HR(Homologous Recombination) of chromosome ends, causing a state known as crisis.[24] The application of these DSB (double strand breaks)repair mechanisms to chromosome ends leads to genetic instability, and while this instability can promote carcinogenesis, it induces apoptosis if experienced for too long.[23] To survive and replicate, precancerous cells must stabilize their telomere lengths. This occurs through telomerase activation or the activation of a telomere-recombination pathway (i.e., the ALT pathway).[22][25] Thus, cancer cells have short telomeres because they progress through an intermediate stage of telomere shortening—caused by division after DNA damage checkpoint inactivation—before enabling mechanisms for maintaining telomere length.
Since the late 1990s, researchers have proposed using telomerase inhibitors as cancer treatments.[26][27][28] While such inhibitors have been seriously considered for cancer therapy since the late 2000s, they are not commonly used.[29] Two concerns with applying telomerase inhibitors in cancer treatment are that effective treatment requires continuous, long-term drug application and that off-target effects are common.[30] For example, the telomerase inhibitor imetelstat, first proposed in 2003,[31][32] has been held up in clinical trials due to hematological toxicity.[30] Despite these concerns, the development of telomerase-based cancer treatments remains an active research area.
Cell cycle timing of telomere elongation
Although telomerase activation does not occur during the cell cycle of normal somatic human cells, the association between telomere elongation (especially elongation by telomerase) and tumor development emphasizes the importance of understanding when such elongation can occur during the cell cycle. Work with S. cerevisiae has identified telomerase activity as restricted to late S phase.[33][34] Researchers generated S. cerevisiae strains with galactose-inducible shortened telomeres.[33] They then used α factor to block cells with induced short telomeres in late G1 phase and measured the change in telomere length when the cells were released under a variety of conditions. They found that when the cells were released and concurrently treated with nocodazole, a G2/M phase cell cycle inhibitor, telomere length increased for the first few hours and then remained constant. In comparison, when cells were released and allowed to cycle, telomere length increased linearly with time.[34] These data suggest that telomere elongation occurs only in S phase. Additional experiments with greater time resolution support this hypothesis and narrow the timeframe to late S phase. Researchers tied telomere elongation in these experiments to telomerase activity by observing that in an S. cerevisiae strain with a dysfunctional ALT pathway telomere elongation still occurs.[34]
References
- ↑ Levy, Michael Z.; Allsopp, Richard C.; Futcher, A.Bruce; Greider, Carol W.; Harley, Calvin B. (June 1996). "Telomere end-replication problem and cell aging". Journal of Molecular Biology 225 (4): 951–960. doi:10.1016/0022-2836(92)90096-3. ISSN 0022-2836. PMID 1613801.
- ↑ Aubert, Geraldine; Lansdorp, Peter M. (April 2008). "Telomeres and Aging". Physiological Reviews 88 (2): 557–579. doi:10.1152/physrev.00026.2007. ISSN 0031-9333. PMID 18391173.
- ↑ Daniali, Lily; Benetos, Athanase; Susser, Ezra; Kark, Jeremy D.; Labat, Carlos; Kimura, Masayuki; Desai, Kunj K.; Granick, Mark et al. (2013-07-24). "Erratum: Corrigendum: Telomeres shorten at equivalent rates in somatic tissues of adults". Nature Communications 4 (1). doi:10.1038/ncomms2976. ISSN 2041-1723.
- ↑ Martínez, Paula; Blasco, Maria A. (2010-09-16). "Role of shelterin in cancer and aging". Aging Cell 9 (5): 653–666. doi:10.1111/j.1474-9726.2010.00596.x. ISSN 1474-9718. PMID 20569239.
- ↑ 5.0 5.1 5.2 5.3 Gobbini, Elisa; Trovesi, Camilla; Cassani, Corinne; Longhese, Maria Pia (28 July 2014). "Telomere uncapping at the crossroad between cell cycle arrest and carcinogenesis". Molecular & Cellular Oncology 1 (1): e29901. doi:10.4161/mco.29901. PMID 27308311.
- ↑ Burma, Sandeep; Chen, Benjamin P.C.; Chen, David J. (September 2006). "Role of non-homologous end joining (NHEJ) in maintaining genomic integrity". DNA Repair 5 (9–10): 1042–1048. doi:10.1016/j.dnarep.2006.05.026. ISSN 1568-7864. PMID 16822724.
- ↑ Ciccia, Alberto; Elledge, Stephen J. (October 2010). "The DNA Damage Response: Making It Safe to Play with Knives". Molecular Cell 40 (2): 179–204. doi:10.1016/j.molcel.2010.09.019. ISSN 1097-2765. PMID 20965415.
- ↑ Murray, Andrew W.; Kirschner, Marc W. (May 1989). "Cyclin synthesis drives the early embryonic cell cycle". Nature 339 (6222): 275–280. doi:10.1038/339275a0. ISSN 0028-0836. PMID 2566917. Bibcode: 1989Natur.339..275M.
- ↑ Ira, Grzegorz; Pellicioli, Achille; Balijja, Alitukiriza; Wang, Xuan; Fiorani, Simona; Carotenuto, Walter; Liberi, Giordano; Bressan, Debra et al. (October 2004). "DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1". Nature 431 (7011): 1011–1017. doi:10.1038/nature02964. ISSN 0028-0836. PMID 15496928. Bibcode: 2004Natur.431.1011I.
- ↑ Ribeyre, Cyril; Shore, David (2012-02-12). "Anticheckpoint pathways at telomeres in yeast". Nature Structural & Molecular Biology 19 (3): 307–313. doi:10.1038/nsmb.2225. ISSN 1545-9993. PMID 22343724.
- ↑ Marcand, S.; Pardo, B.; Gratias, A.; Cahun, S.; Callebaut, I. (2008-05-01). "Multiple pathways inhibit NHEJ at telomeres". Genes & Development 22 (9): 1153–1158. doi:10.1101/gad.455108. ISSN 0890-9369. PMID 18451106.
- ↑ Bonetti, Diego; Clerici, Michela; Anbalagan, Savani; Martina, Marina; Lucchini, Giovanna; Longhese, Maria Pia (2010-05-27). "Shelterin-Like Proteins and Yku Inhibit Nucleolytic Processing of Saccharomyces cerevisiae Telomeres". PLOS Genetics 6 (5): e1000966. doi:10.1371/journal.pgen.1000966. ISSN 1553-7404. PMID 20523746.
- ↑ Hockemeyer, Dirk; Sfeir, Agnel J; Shay, Jerry W; Wright, Woodring E; de Lange, Titia (2005-06-23). "POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end". The EMBO Journal 24 (14): 2667–2678. doi:10.1038/sj.emboj.7600733. ISSN 0261-4189. PMID 15973431.
- ↑ Wu, Ling; Multani, Asha S.; He, Hua; Cosme-Blanco, Wilfredo; Deng, Yu; Deng, Jian Min; Bachilo, Olga; Pathak, Sen et al. (July 2006). "Pot1 Deficiency Initiates DNA Damage Checkpoint Activation and Aberrant Homologous Recombination at Telomeres". Cell 126 (1): 49–62. doi:10.1016/j.cell.2006.05.037. ISSN 0092-8674. PMID 16839876.
- ↑ Sfeir, A.; Kabir, S.; van Overbeek, M.; Celli, G. B.; de Lange, T. (2010-03-25). "Loss of Rap1 Induces Telomere Recombination in the Absence of NHEJ or a DNA Damage Signal". Science 327 (5973): 1657–1661. doi:10.1126/science.1185100. ISSN 0036-8075. PMID 20339076. Bibcode: 2010Sci...327.1657S.
- ↑ Karlseder, J. (1999-02-26). "p53- and ATM-Dependent Apoptosis Induced by Telomeres Lacking TRF2". Science 283 (5406): 1321–1325. doi:10.1126/science.283.5406.1321. ISSN 0036-8075. PMID 10037601.
- ↑ Celli, Giulia B.; de Lange, Titia (2005-06-19). "DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion". Nature Cell Biology 7 (7): 712–718. doi:10.1038/ncb1275. ISSN 1465-7392. PMID 15968270.
- ↑ Doksani, Ylli; Wu, John Y.; de Lange, Titia; Zhuang, Xiaowei (October 2013). "Super-Resolution Fluorescence Imaging of Telomeres Reveals TRF2-Dependent T-loop Formation". Cell 155 (2): 345–356. doi:10.1016/j.cell.2013.09.048. ISSN 0092-8674. PMID 24120135.
- ↑ von Zglinicki, Thomas (July 2002). "Oxidative stress shortens telomeres". Trends in Biochemical Sciences 27 (7): 339–344. doi:10.1016/s0968-0004(02)02110-2. ISSN 0968-0004. PMID 12114022.
- ↑ Shay, Jerry W.; Wright, Woodring E. (December 2011). "Role of telomeres and telomerase in cancer". Seminars in Cancer Biology 21 (6): 349–353. doi:10.1016/j.semcancer.2011.10.001. ISSN 1044-579X. PMID 22015685.
- ↑ Wang, Haiyong; Zhang, Xiaoshan; Teng, Lisong; Legerski, Randy J. (June 2015). "DNA damage checkpoint recovery and cancer development". Experimental Cell Research 334 (2): 350–358. doi:10.1016/j.yexcr.2015.03.011. ISSN 0014-4827. PMID 25842165.
- ↑ 22.0 22.1 Chin, Lynda; Artandi, Steven E; Shen, Qiong; Tam, Alice; Lee, Shwu-Luan; Gottlieb, Geoffrey J; Greider, Carol W; DePinho, Ronald A (May 1999). "p53 Deficiency Rescues the Adverse Effects of Telomere Loss and Cooperates with Telomere Dysfunction to Accelerate Carcinogenesis". Cell 97 (4): 527–538. doi:10.1016/s0092-8674(00)80762-x. ISSN 0092-8674. PMID 10338216.
- ↑ 23.0 23.1 Rudolph, Karl Lenhard; Millard, Melissa; Bosenberg, Marcus W.; DePinho, Ronald A. (June 2001). "Telomere dysfunction and evolution of intestinal carcinoma in mice and humans". Nature Genetics 28 (2): 155–159. doi:10.1038/88871. ISSN 1061-4036. PMID 11381263.
- ↑ Counter, C.M.; Avilion, A.A.; LeFeuvre, C.E.; Stewart, N.G.; Greider, C.W.; Harley, C.B.; Bacchetti, S. (May 1992). "Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity.". The EMBO Journal 11 (5): 1921–1929. doi:10.1002/j.1460-2075.1992.tb05245.x. ISSN 0261-4189. PMID 1582420.
- ↑ Lundblad, Victoria; Blackburn, Elizabeth H. (April 1993). "An alternative pathway for yeast telomere maintenance rescues est1− senescence". Cell 73 (2): 347–360. doi:10.1016/0092-8674(93)90234-h. ISSN 0092-8674. PMID 8477448.
- ↑ Saretzki, Gabriele (May 2003). "Telomerase inhibition as cancer therapy". Cancer Letters 194 (2): 209–219. doi:10.1016/s0304-3835(02)00708-5. ISSN 0304-3835. PMID 12757979.
- ↑ Kim, N.W. (April 1997). "Clinical implications of telomerase in cancer". European Journal of Cancer 33 (5): 781–786. doi:10.1016/s0959-8049(97)00057-9. ISSN 0959-8049. PMID 9282117.
- ↑ Autexier, Chantal; Greider, Carol (October 1996). "Telomerase and cancer: revisiting the telomere hypothesis". Trends in Biochemical Sciences 21 (10): 387–391. doi:10.1016/s0968-0004(96)10042-6. ISSN 0968-0004. PMID 8918193.
- ↑ Ivancich, Marko; Schrank, Zachary; Wojdyla, Luke; Leviskas, Brandon; Kuckovic, Adijan; Sanjali, Ankita; Puri, Neelu (2017-02-19). "Treating Cancer by Targeting Telomeres and Telomerase". Antioxidants 6 (1): 15. doi:10.3390/antiox6010015. ISSN 2076-3921. PMID 28218725.
- ↑ 30.0 30.1 Jafri, Mohammad A.; Ansari, Shakeel A.; Alqahtani, Mohammed H.; Shay, Jerry W. (2016-06-20). "Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies". Genome Medicine 8 (1): 69. doi:10.1186/s13073-016-0324-x. ISSN 1756-994X. PMID 27323951.
- ↑ Gryaznov, Sergei; Asai, Akira; Oshima, Yuko; Yamamoto, Yoshihiro; Pongracz, Krisztina; Pruzan, Ronald; Wunder, Ellen; Piatyszek, Mieczyslaw et al. (October 2003). "Oligonucleotide N3′ → P5′ Thio-phosphoramidate Telomerase Template Antagonists as Potential Anticancer Agents". Nucleosides, Nucleotides and Nucleic Acids 22 (5–8): 577–581. doi:10.1081/NCN-120021958. PMID 14565232.
- ↑ Röth, Alexander; Harley, Calvin B.; Baerlocher, Gabriela M. (2010). "Imetelstat (GRN163L) - Telomerase-Based Cancer Therapy". Small Molecules in Oncology. Recent Results in Cancer Research. 184. pp. 221–234. doi:10.1007/978-3-642-01222-8_16. ISBN 978-3-642-01221-1.
- ↑ 33.0 33.1 Marcand, S.; Brevet, V; Gilson, E (15 June 1999). "Progressive cis-inhibition of telomerase upon telomere elongation". The EMBO Journal 18 (12): 3509–3519. doi:10.1093/emboj/18.12.3509. PMID 10369690.
- ↑ 34.0 34.1 34.2 Marcand, Stéphane; Brevet, Vanessa; Mann, Carl; Gilson, Eric (April 2000). "Cell cycle restriction of telomere elongation". Current Biology 10 (8): 487–490. doi:10.1016/s0960-9822(00)00450-4. PMID 10801419.
External links
- Irving, Michael (2021-11-10). "Anti-aging research uncovers new role for telomeres" (in en-US). https://newatlas.com/biology/anti-aging-telomeres-genome-damage/.
 |

