Biology:Ranavirus
| Ranavirus | |
|---|---|
| |
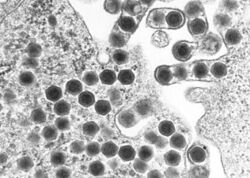
| Transmission electron micrograph of ranaviruses (dark hexagons) gathering at the cell border and leaving the cell via a process called "budding". | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Varidnaviria |
| Kingdom: | Bamfordvirae |
| Phylum: | Nucleocytoviricota |
| Class: | Megaviricetes |
| Order: | Pimascovirales |
| Family: | Iridoviridae |
| Subfamily: | Alphairidovirinae |
| Genus: | Ranavirus |
Ranavirus is a genus of viruses in the family Iridoviridae.[1] There are six other genera of viruses within the family Iridoviridae, but Ranavirus is the only one that includes viruses that are infectious to amphibians and reptiles. Additionally, it is one of the three genera within this family which infect teleost fishes, along with Lymphocystivirus and Megalocytivirus.[2]
Ecological impact
The Ranaviruses, like the Megalocytiviruses, are an emerging group of closely related dsDNA viruses which cause systemic infections in a wide variety of wild and cultured fresh and saltwater fishes. As with Megalocytiviruses, Ranavirus outbreaks are therefore of considerable economic importance in aquaculture, as epizootics can result in moderate fish loss or mass mortality events of cultured fishes. Unlike Megalocytiviruses, however, Ranavirus infections in amphibians have been implicated as a contributing factor in the global decline of amphibian populations.[3][4] The impact of Ranaviruses on amphibian populations has been compared to the chytrid fungus Batrachochytrium dendrobatidis, the causative agent of chytridiomycosis.[5][6][7] In the UK, the severity of disease outbreaks is thought to have increased due to climate change.[8]
Etymology
Rana is derived from the Latin for "frog",[9] reflecting the first isolation of a Ranavirus in 1960s from the Northern leopard frog (Lithobates pipiens).[10][11][12]
Evolution
File:Reptiles, Amphibians in US Succumbing to Deadly Ranavirus.ogv The ranaviruses appear to have evolved from a fish virus which subsequently infected amphibians and reptiles.[13]
Hosts
Anuran hosts
- Wood frogs (Lithobates sylvaticus)
- American Bullfrog (Lithobates catesbieanus)
- Pickerel Frog
Urodelan hosts
Reptilian hosts
- Green pythons (Chondropython viridis)[14]
- Burmese star tortoises (Geochelone platynota)
- Leopard tortoise (Geochelone pardalis)[15]
- Gopher tortoises (Gopherus polyphemus)
- Mountain lizard (Lacerta monticola)[16]
- Eastern box turtles (Terrapene carolina carolina)[17]
- Florida box turtles (Terrapene carolina bauri)
- Western ornate box turtles (Terrapene ornata)[18]
- Spur-thighed tortoises (Testudo graeca)[19]
- Hermann's tortoises (Testudo hermanni)
- Egyptian tortoises (Testudo kleinmanni)
- Russian tortoises (Testudo horsfieldii)
- Marginated tortoises (Testudo marginata)
- Red-eared sliders (Trachemys scripta elegans)[18]
- Common snapping turtles (Chelydra serpentina)[20]
- Chinese softshell turtles (Pelodiscus sinensis)[21]
- Common flat-tail gecko (Uroplatus fimbriatus)[22]
- Eastern Fence Lizard (Sceloporus undulatus) [23]
Taxonomy
The genus contains the following species:[24]
- Ambystoma tigrinum virus
- Common midwife toad virus
- Epizootic haematopoietic necrosis virus
- European North Atlantic ranavirus
- Frog virus 3
- Santee-Cooper ranavirus
- Singapore grouper iridovirus
The family Iridoviridae is divided into seven genera which include Chloriridovirus, Iridovirus, Lymphocystivirus, Megalocytivirus, and Ranavirus.[1] The genus Ranavirus contains three viruses known to infect amphibians (Ambystoma tigrinum virus (ATV), Bohle iridovirus (BIV), and frog virus 3).[25]
Structure
Ranaviruses are large icosahedral DNA viruses measuring approximately 150 nm in diameter with a large single linear dsDNA genome of roughly 105 kbp[26] which codes for around 100 gene products.[27] The main structural component of the protein capsid is the major capsid protein (MCP).
| Genus | Structure | Symmetry | Capsid | Genomic arrangement | Genomic segmentation |
|---|---|---|---|---|---|
| Ranavirus | Polyhedral | T=133 or 147 | Linear | Monopartite |
Replication
Ranaviral replication is well studied using Frog virus 3 (FV3).[25][26] Replication of FV3 occurs between 12 and 32 degrees Celsius.[27] Ranaviruses enter the host cell by receptor-mediated endocytosis.[28] Viral particles are uncoated and subsequently move into the cell nucleus, where viral DNA replication begins via a virally encoded DNA polymerase.[29] Viral DNA then abandons the cell nucleus and begins the second stage of DNA replication in the cytoplasm, ultimately forming DNA concatemers.[29] The viral DNA is then packaged via a headful mechanism into infectious virions.[25] The ranavirus genome, like other iridoviral genomes is circularly permuted and exhibits terminally redundant DNA.[29] There is evidence that ranavirus infections target macrophages as a mechanism for gaining entry to cells. [30]
| Genus | Host details | Tissue tropism | Entry details | Release details | Replication site | Assembly site | Transmission |
|---|---|---|---|---|---|---|---|
| Ranavirus | Frogs; snakes | None | Cell receptor endocytosis | Lysis; budding | Nucleus | Cytoplasm | Contact |
DNA repair
Andrias davidianus ranavirus, isolated from the Chinese giant salamander, encodes a protein (Rad2 homolog) that has a key role in the repair of DNA by homologous recombination and in DNA double-strand break repair.[31]
Transmission
Transmission of ranaviruses is thought to occur by multiple routes, including contaminated soil, direct contact, waterborne exposure, and ingestion of infected tissues during predation, necrophagy or cannibalism.[11][32] Ranaviruses are relatively stable in aquatic environments, persisting several weeks or longer outside a host organism.[11]
Epizoology
Amphibian mass mortality events due to Ranavirus have been reported in Asia, Europe, North America, and South America.[11] Ranaviruses have been isolated from wild populations of amphibians in Australia, but have not been associated with mass mortality on that continent.[11][33][34]
Pathogenesis
Synthesis of viral proteins begins within hours of viral entry[27] with necrosis or apoptosis occurring as early as a few hours post infection.[26][35]
Seasonal disease dynamics
There are several hypotheses for seasonal outbreak patterns observed for Ranavirosis mortality events.[36] Ranaviruses grow in vitro between 8-30 °C, however for most isolates, warmer temperature result in faster viral replication.[36] A combination of this optimal growth temperature along with shifts in larval amphibian susceptibility result in seasonal outbreak events most often observed during warm summer months.[37] Amphibian mortality events are often observed as larval amphibians reach late Gosner stages approaching metamorphosis.[38] As larval amphibians reach metamorphic stages of development, their immune system is reorganized prior to the development of adult tissues.[39] During this time period, amphibians are stressed, and their immune systems are down regulated. This decrease in immune function and warmer environmental temperatures allows for greater viral replication and cellular damage to occur. Across 64 mortality events in the United States 54% were found to occur between June-August.[37]
Environmental persistence
The environmental persistence of Ranaviruses is not understood well, however in realistic environmental conditions the T90 value of an FV3-like virus is 1 day.[40] The duration of persistence is likely affected by temperature and microbial conditions. It is unlikely that ranaviruses persist in the environment outside of host species between outbreak events. Researchers have explored several pathogen reservoirs for the virus which might explain how the virus can persist within an amphibian community. In some amphibian populations, sub-clinically infected individuals may serve as reservoirs for the pathogen.[6] These sub-clinically infected individuals are responsible for reintroduction of the virus to the larval population. With ranaviruses being capable of infected multiple taxa, and with there being differences in susceptibility between taxa, it is likely that sympatric fish and reptile species may serve as reservoirs for virus as well. Interclass transmission has been proven through the use of mesocosm studies.[41]
Gross pathology
Gross lesions associated with Ranavirus infection include erythema, generalized swelling, hemorrhage, limb swelling, and swollen and friable livers.[11]
See also
References
- ↑ 1.0 1.1 "Iridoviridae". https://ictv.global/report/chapter/iridoviridae/iridoviridae.
- ↑ Whittington, RJ; Becker, JA; Dennis, MM (2010). "Iridovirus infections in finfish – critical review with emphasis on ranaviruses". Journal of Fish Diseases 33 (2): 95–122. doi:10.1111/j.1365-2761.2009.01110.x. PMID 20050967.
- ↑ Teacher, A. G. F.; Cunningham, A. A.; Garner, T. W. J. (2010-06-10). "Assessing the long-term impact of Ranavirus infection in wild common frog populations: Impact of Ranavirus on wild frog populations". Animal Conservation 13 (5): 514–522. doi:10.1111/j.1469-1795.2010.00373.x.
- ↑ Price, Stephen J.; Garner, Trenton W.J.; Nichols, Richard A.; Balloux, François; Ayres, César; Mora-Cabello de Alba, Amparo; Bosch, Jaime (November 2014). "Collapse of Amphibian Communities Due to an Introduced Ranavirus". Current Biology 24 (21): 2586–2591. doi:10.1016/j.cub.2014.09.028. PMID 25438946.
- ↑ Jancovich, James K; Mao, Jinghe; Chinchar, V.Gregory; Wyatt, Christopher; Case, Steven T; Kumar, Sudhir; Valente, Graziela; Subramanian, Sankar et al. (2003). "Genomic sequence of a ranavirus (family Iridoviridae) associated with salamander mortalities in North America". Virology 316 (1): 90–103. doi:10.1016/j.virol.2003.08.001. PMID 14599794.
- ↑ 6.0 6.1 Brunner, Jesse L.; Schock, Danna M.; Davidson, Elizabeth W.; Collins, James P. (2004). "Intraspecific Reservoirs: Complex Life History and the Persistence of a Lethal Ranavirus". Ecology 85 (2): 560. doi:10.1890/02-0374.
- ↑ Pearman, Peter B.; Garner, Trenton W. J. (2005). "Susceptibility of Italian agile frog populations to an emerging strain of Ranavirus parallels population genetic diversity". Ecology Letters 8 (4): 401. doi:10.1111/j.1461-0248.2005.00735.x.
- ↑ Price, Stephen J.; Leung, William T. M.; Owen, Christopher J.; Puschendorf, Robert; Sergeant, Chris; Cunningham, Andrew A.; Balloux, Francois; Garner, Trenton W. J. et al. (2019-05-09). "Effects of historic and projected climate change on the range and impacts of an emerging wildlife disease". Global Change Biology 25 (8): 2648–2660. doi:10.1111/gcb.14651. ISSN 1354-1013. PMID 31074105. Bibcode: 2019GCBio..25.2648P.
- ↑ Harper, Douglas. "frog". Online Etymology Dictionary. https://www.etymonline.com/?term=frog.
- ↑ Granoff, A; Came, PE; Rafferty, KA (1965). "The isolation and properties of viruses from Rana pipiens: their possible relationship to the renal adenocarcinoma of the leopard frog". Annals of the New York Academy of Sciences 126 (1): 237–255. doi:10.1111/j.1749-6632.1965.tb14278.x. PMID 5220161. Bibcode: 1965NYASA.126..237G.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 Gray, MJ; Miller, DL; Hoverman, JT (2009). "Ecology and pathology of amphibian ranaviruses". Diseases of Aquatic Organisms 87 (3): 243–266. doi:10.3354/dao02138. PMID 20099417.
- ↑ Rafferty, KA (1965). "The cultivation of inclusion-associated viruses from Lucke tumor frogs". Annals of the New York Academy of Sciences 126 (1): 3–21. doi:10.1111/j.1749-6632.1965.tb14266.x. PMID 5220167. Bibcode: 1965NYASA.126....3R.
- ↑ Jancovich, JK; Bremont, M; Touchman, JW; Jacobs, BL (2010). "Evidence for multiple recent host species shifts among the Ranaviruses (family Iridoviridae)". J Virol 84 (6): 2636–2647. doi:10.1128/JVI.01991-09. PMID 20042506.
- ↑ First identification of a ranavirus from green pythons (Chondropython viridis); Williamson; Coupar; Middleton; Hengstberger; Gould; Selleck; Wise et al. (2002). "First identification of a ranavirus from green pythons (Chondropython viridis)". Journal of Wildlife Diseases 38 (2): 239–52. doi:10.7589/0090-3558-38.2.239. PMID 12038121.
- ↑ Benetka V. (2007). "First report of an iridovirus (genus Ranavirus) infection in a leopard tortoise (Geochelone pardalis pardalis)". Vet Med Austria 94: 243–248. http://www.schildkroeten-sfb.ch/fileadmin/docs/news/729-pantherschildkroete.pdf.
- ↑ De Matos, A. P.; Caeiro, M. F.; Papp, T; Matos, B. A.; Correia, A. C.; Marschang, R. E. (2011). "New viruses from Lacerta monticola (Serra da Estrela, Portugal): Further evidence for a new group of nucleo-cytoplasmic large deoxyriboviruses (NCLDVs)". Microscopy and Microanalysis 17 (1): 101–8. doi:10.1017/S143192761009433X. PMID 21138619. Bibcode: 2011MiMic..17..101A.
- ↑ Mao, J; Hedrick, RP; Chinchar, VG (1997). "Molecular characterization, sequence analysis, and taxonomic position of newly isolated fish iridoviruses". Virology 229 (1): 212–220. doi:10.1006/viro.1996.8435. PMID 9123863.
- ↑ 18.0 18.1 Johnson, A. J.; Pessier, A. P.; Jacobson, E. R. (2007). "Experimental transmission and induction of ranaviral disease in Western Ornate box turtles (Terrapene ornata ornata) and red-eared sliders (Trachemys scripta elegans)". Veterinary Pathology 44 (3): 285–97. doi:10.1354/vp.44-3-285. PMID 17491069.
- ↑ Blahak S., Uhlenbrok C. "Ranavirus infections in European terrestrial tortoises in Germany". Proceedings of the 1st International Conference on Reptile and Amphibian Medicine; Munich, Germany. 4–7 March 2010; pp. 17–23
- ↑ McKenzie, C. M.; Piczak, M.L.; Snyman, H. N.; Joseph, T.; Theijin, C.; Chow-Fraser, P.; Jardine, C. M. (2019). "First report of ranavirus mortality in a common snapping turtle Chelydra serpentina". Diseases of Aquatic Organisms 132 (3): 221–227. doi:10.3354/dao03324. PMID 31188138. https://www.int-res.com/articles/dao2018/132/d132p221.pdf.
- ↑ Chen, Z. X.; Zheng, J. C.; Jiang, Y. L. (1999). "A new iridovirus isolated from soft-shelled turtle". Virus Research 63 (1–2): 147–51. doi:10.1016/S0168-1702(99)00069-6. PMID 10509727.
- ↑ Marschang, R. E.; Braun, S; Becher, P (2005). "Isolation of a ranavirus from a gecko (Uroplatus fimbriatus)". Journal of Zoo and Wildlife Medicine 36 (2): 295–300. doi:10.1638/04-008.1. PMID 17323572.
- ↑ Goodman, R.; Hargadon, K; Carter, E. (2018). "Detection of Ranavirus in Eastern Fence Lizards and Eastern Box Turtles in Central Virginia". Northeastern Naturalist 25 (3): 391–398. doi:10.1656/045.025.0306.
- ↑ "Virus Taxonomy: 2020 Release". International Committee on Taxonomy of Viruses (ICTV). March 2021. https://ictv.global/taxonomy.
- ↑ 25.0 25.1 25.2 Chinchar VG, Essbauer S, He JG, Hyatt A, Miyazaki T, Seligy V, Williams T (2005). "Family Iridoviridae" pp. 145–162 in Fauquet CM, Mayo MA, Maniloff J, Desselburger U, Ball LA (eds). Virus Taxonomy, Eighth report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, USA.
- ↑ 26.0 26.1 26.2 Williams T, Barbosa-Solomieu V, Chinchar GD (2005). "A decade of advances in iridovirus research" 173-148. In Maramorosch K, Shatkin A (eds). Advances in virus research, Vol. 65 Academic Press, New York, USA.
- ↑ 27.0 27.1 27.2 Chinchar, VG (2002). "Ranaviruses (family Iridoviridae) emerging cold-blooded killers". Archives of Virology 147 (3): 447–470. doi:10.1007/s007050200000. PMID 11958449.
- ↑ Eaton, Heather E.; Ring, Brooke A.; Brunetti, Craig R. (2010). "The genomic diversity and phylogenetic relationship in the family Iridoviridae". Viruses 2 (7): 1458–75. doi:10.3390/v2071458. PMID 21994690.
- ↑ 29.0 29.1 29.2 Goorha, R (1982). "Frog virus 3 DNA replication occurs in two stages". Journal of Virology 43 (2): 519–28. doi:10.1128/JVI.43.2.519-528.1982. PMID 7109033.
- ↑ Girdhar, Khyati; Powis, Amaya; Raisingani, Amol; Chrudinová, Martina; Huang, Ruixu; Tran, Tu; Sevgi, Kaan; Dogus Dogru, Yusuf et al. (29 September 2021). "Viruses and Metabolism: The Effects of Viral Infections and Viral Insulins on Host Metabolism". Annual Review of Virology 8 (1): 373–391. doi:10.1146/annurev-virology-091919-102416. ISSN 2327-056X. PMID 34586876.
- ↑ Ke F, Zhang QY. ADRV 12L: A Ranaviral Putative Rad2 Family Protein Involved in DNA Recombination and Repair. Viruses. 2022 Apr 27;14(5):908. doi: 10.3390/v14050908. PMID: 35632650; PMCID: PMC9146916
- ↑ Brenes, Roberto; Gray, Matthew J.; Waltzek, Thomas B.; Wilkes, Rebecca P.; Miller, Debra L. (25 March 2014). "Transmission of Ranavirus between Ectothermic Vertebrate Hosts" (in en). PLOS ONE 9 (3): e92476. doi:10.1371/journal.pone.0092476. ISSN 1932-6203. PMID 24667325. Bibcode: 2014PLoSO...992476B.
- ↑ Speare, R; Smith, JR (1992). "An iridovirus-like agent isolated from the ornate burrowing frog Limnodynastes ornatus in northern Australia". Diseases of Aquatic Organisms 14: 51–57. doi:10.3354/dao014051.
- ↑ Cullen, BR; Owens, L (2002). "Experimental challenge and clinical cases of Bohle iridovirus (BIV) in native Australian anurans". Diseases of Aquatic Organisms 49 (2): 83–92. doi:10.3354/dao049083. PMID 12078986.
- ↑ Chinchar, VG; Bryan, L; Wang, J; Long, S; Chinchar, GD (2003). "Induction of apoptosis in frog virus 3-infected cells". Virology 306 (2): 303–312. doi:10.1016/S0042-6822(02)00039-9. PMID 12642103.
- ↑ 36.0 36.1 Brunner, Jesse L; Storfer, Andrew; Gray, Matthew J; Hoverman, Jason T (2015). Ranaviruses: Lethal Pathogens of Ectothermic Vertebrates. New York: Springer. p. 71-104. doi:10.1007/978-3-319-13755-1_4. ISBN 978-3-319-13755-1.
- ↑ 37.0 37.1 Green, D E; Converse, K A; Schrader, A K (2002). "Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996-2001". Domestic Animal/Wildlife Interface: Issues for Disease Control, Conservation, Sustainable Food Production, and Emerging Diseases 969 (1): 323–339. doi:10.1111/j.1749-6632.2002.tb04400.x. PMID 12381613. Bibcode: 2002NYASA.969..323G.
- ↑ Green, D E; Converse, K A (2005). "Diseases of frogs and toads". Wildlife Diseases: Landscape Epidemiology, Spatial Distribution, and Utilization of Remote Sensing Technology.: 89-117. http://pubs.er.usgs.gov/publication/85615.
- ↑ Rollins-Smith, L A (1998). "Metamorphosis and the amphibian immune system". Immunological Reviews 166: 221–230. doi:10.1111/j.1600-065X.1998.tb01265.x. PMID 9914915.
- ↑ Johnson, A F; Brunner, J L (2014). "Persistence of an amphibian ranavirus in aquatic communities". Diseases of Aquatic Organisms 111 (2): 129–138. doi:10.3354/dao02774. PMID 25266900.
- ↑ Brenes, Roberto; Gray, MJ; Waltzek, TB; Wilkes, RP; Miller, DL (2014). "Transmission of Ranavirus between Ectothermic Vertebrate Hosts". PLOS ONE 9 (3): e92476. doi:10.1371/journal.pone.0092476. PMID 24667325. Bibcode: 2014PLoSO...992476B.
External links
- ICTV Online (10th) Report: Iridoviridae
- Viralzone: Ranavirus
- Global Ranavirus Consortium
- Viral Diseases of Amphibians
- More information on Ranavirus and other pathogens impacting amphibian populations, including Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans can be found at the Southeast Partners in Amphibian and Reptile Conservation disease task team web-page. [1]
Wikidata ☰ Q3418768 entry
 |

