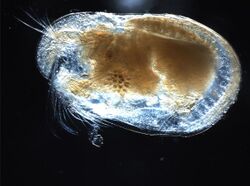
Biology:Ostracod
| Ostracod | |
|---|---|
| |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Superclass: | Oligostraca |
| Class: | Ostracoda Latreille, 1802 |
| Subclasses and orders | |
| |
Ostracods, or ostracodes, are a class of the Crustacea (class Ostracoda), sometimes known as seed shrimp. Some 33,000 species (only 13,000 of which are extant) have been identified,[2] grouped into 7 valid orders.[2] They are small crustaceans, typically around 1 mm (0.04 in) in size, but varying from 0.2 to 30 mm (0.008 to 1 in) in the case of the marine Gigantocypris. The largest known freshwater species is Megalocypris princeps, which reach 8mm in length.[3][4] In most cases, their bodies are flattened from side to side and protected by a bivalve-like valve or "shell" made of chitin, and often calcium carbonate. The family Entocytheridae and many planktonic forms do not have calcium carbonate.[5][6] The hinge of the two valves is in the upper (dorsal) region of the body. Ostracods are grouped together based on shell and soft part morphology. While early work indicated the group may not be monophyletic[7] and early molecular phylogeny was ambiguous on this front,[8] recent combined analyses of molecular and morphological data suggested monophyly in analyses with broadest taxon sampling, but this monophyly had no or very little support (Fig. 1 - bootstrap 0, 17 and 46, often values above 95 are considered sufficient for the taxon support).[9] They have a wide range of diets, and the class includes carnivores, herbivores, scavengers and filter feeders, but most ostracods are deposit feeders.
Etymology
Ostracod comes from the Greek óstrakon meaning shell or tile.
Fossils

Ostracods are "by far the most common arthropods in the fossil record"[10] with fossils being found from the early Ordovician to the present. An outline microfaunal zonal scheme based on both Foraminifera and Ostracoda was compiled by M. B. Hart.[11] Freshwater ostracods have even been found in Baltic amber of Eocene age, having presumably been washed onto trees during floods.[12]
Ostracods have been particularly useful for the biozonation of marine strata on a local or regional scale, and they are invaluable indicators of paleoenvironments because of their widespread occurrence, small size, easily preservable, generally moulted, calcified bivalve carapaces; the valves are a commonly found microfossil.
A find in Queensland, Australia in 2013, announced in May 2014, at the Bicentennary Site in the Riversleigh World Heritage area, revealed both male and female specimens with very well preserved soft tissue. This set the Guinness World Record for the oldest penis.[13] Males had observable sperm that is the oldest yet seen and, when analysed, showed internal structures and has been assessed as being the largest sperm (per body size) of any animal recorded. It was assessed that the fossilisation was achieved within several days, due to phosphorus in the bat droppings of the cave where the ostracods were living.[14]
Description
The body of an ostracod is encased by a carapace originating from the head region, and consists of two valves superficially resembling the shell of a clam. A distinction is made between the valve (hard parts) and the body with its appendages (soft parts). Studies of the embryonic development in Myodocopida shows that the bivalved carapace developes from two independent buds of the carapace valves. As the two halves grows, they meet in the middle. In Manawa, an ostracod in the order Palaeocopida, the carapace originates as a single element and during growth folds at the midline.[15][16]
Body parts
File:Ostracod swimming motions 20200520.gif The body consists of a head and thorax, separated by a slight constriction. Unlike many other crustaceans, the body is not clearly divided into segments. Most species have completely or partly lost their trunk segmentation, and there are no boundaries between the thorax and abdomen, and it has therefore been impossible to tell if the first pair of limbs after the maxillae belongs to the head or the thorax. With a few exception, like platycopids which have an 11-segmented trunk, the abdomen in ostracods has no visible segments.[17]
The head is the largest part of the body, and bears four pairs of appendages. Two pairs of well-developed antennae are used to swim through the water. In addition, there are a pair of mandibles and a pair of maxillae. The thorax has three primary pairs of appendages. The first of these has different functions in different groups. It can be used for feeding (Cypridoidea) or for walking (Cytheroidea), and in some species it has evolved into a male clasping organ. The second pair is mainly used for locomotion, and the third is used for walking or cleaning, but can also be reduced or absent. Both the second and third pair are absent in suborder Cladocopina.[18] In the Myodocopina the third pair is a multisegmented cleaning organ that resembles a worm. Their external genitals seem to originate from the fusion of three to five appendages. The two "rami", or projections, from the tip of the tail point downward and slightly forward from the rear of the shell.[19][20][21]:40
All ostracods have a pair of "ventilatory appendages" that is beating rhythmically, which creates a water current between the body and the inner surface of the carapace. Podocopa, the largest subclass, have no gills, heart or circulatory system, so the gas exchange take place all over the surface. The other subclass of ostracods, the Myodocopa, do have a heart, and the family Cylindroleberididae also have 6-8 lamellar gills. Certain other larger members of Myodocopa, even if they don't have gills, have a circulatory system where hemolymph sinuses absorbs oxygen through special areas on the inner wall of the carapace.[22][23] In addition, the respiratory protein hemocyanin has been found in the two orders Myodocopida and Platycopida.[24] Nitrogenous waste is excreted through glands on the maxillae, antennae, or both.[19]
The primary sense of ostracods is likely touch, as they have several sensitive hairs on their bodies and appendages. Compound eyes are only found in Myodocopina within the Myodocopa.[25] The order Halocyprida in the same subclass is eyeless.[26] Podocopid ostracods have just a naupliar eye consisting of two lateral ocelli and a single ventral ocellus, but the ventral one is absent in some species.[19][27][28] Platycopida was assumed to be completely eyeless, but two species, Keijcyoidea infralittoralis and Cytherella sordida, have been found to both possess a nauplius eye too.[29]
Palaeoclimatic reconstruction
A new method is in development called mutual ostracod temperature range (MOTR), similar to the mutual climatic range (MCR) used for beetles, which can be used to infer palaeotemperatures.[30] The ratio of oxygen-18 to oxygen-16 (δ18O) and the ratio of magnesium to calcium (Mg/Ca) in the calcite of ostracod valves can be used to infer information about past hydrological regimes, global ice volume and water temperatures.
Distribution
Ecologically, marine ostracods can be part of the zooplankton or (most commonly) are part of the benthos, living on or inside the upper layer of the sea floor. Ostracods has been found as deep as 9,307 m (genus Krithe in family Krithidae).[31] Subclass Myodocopa and the two podocop orders Palaeocopida and Platycopida are restricted to marine environments (except for Platycopida which have a few brackish species)[32],[33][34] but we find non-marine forms in the four superfamilies Terrestricytheroidea, Cypridoidea, Darwinuloidea, and Cytheroidea in the order Podocopida. Terrestricytheroidea is semi-terrestrial and usually found in brackish and marine-influenced environments such as salt marshes, but not in freshwater.[35] The other three superfamilies also live in freshwater (Darwinuloidea is exclusively non-marine).[36][37][38] Of these three, only Cypridoidea have freshwater species able to swim.[39] Representatives living in terrestrial habitats are also found in all three freshwater groups,[40] such as genus Mesocypris which is known from humid forest soils of South Africa , Australia and New Zealand.[41]
As of 2008, around 2000 species and 200 genera of non-marine ostracods are found.[42] However, a large portion of diversity is still undescribed, indicated by undocumented diversity hotspots of temporary habitats in Africa and Australia.[43] Non-marine species have been found to live in sulfidic cave ecosystems such as the Movile Cave, deep groundwaters, hypersaline waters, acidic waters with pH as low as 3.4, phytotelmata in plants like bromeliads, and in temperatures varying from almost freezing to more than 50 °C in hot springs.[44][45][46] Of the known specific and generic diversity of non-marine ostracods, half (1000 species, 100 genera) belongs to one family (of 13 families), Cyprididae.[43] Many Cyprididae occur in temporary water bodies and have drought-resistant eggs, mixed/parthenogenetic reproduction, and the ability to swim. These biological attributes preadapt them to form successful radiations in these habitats.[47]
Ecology
Lifecycle
Male ostracods have two penises, corresponding to two genital openings (gonopores) on the female. The individual sperm are often large, and are coiled up within the testis prior to mating; in some cases, the uncoiled sperm can be up to six times the length of the male ostracod itself. Mating typically occurs during swarming, with large numbers of females swimming to join the males. Some species are partially or wholly parthenogenetic.[19] Superfamily Darwinuloidea was assumed to have reproduced asexually for the last 200 million years, but rare males have since been discovered in one of the species.[48][49]
In the subclass Myodocopa, all members of the order Myodocopida have brood care, releasing their offspring as first instars, allowing a pelagic lifestyle. In the order Halocyprida the eggs are released directly into the sea, except for a single genus with brood care. In the subclass Podocopa, brood care is only found in Darwinulocopina and some Cytherocopina in the order Podocopida. In the remaining Podocopa it is common to glue the eggs to a firm surface, like vegetation or the substratum. These eggs are often resting eggs, and remain dormant during desiccation and extreme temperatures, only hatching when exposed to more favorable conditions.[50][51] Species adapted to vernal pools can reach sexual maturity in just 30 days after hatching.[52] There is no larval stage or metamorphosis (direct development). Instead they hatch from the egg as juveniles with the bivalved carapace and at least three functional limbs. As the juvenile grows through a series of molts they acquire more limbs and develop further the already existing ones.[53] They reach sexual maturity in the final instar and then never molts again. The number of instars they go through before adulthood varies. In Podocopa it is eight or nine (but family Entocytheridae and suborder Bairdiocopina has only seven),[54] the Halocyprida goes through six or seven, and Myodocopida only four to six. They are able to produce several offspring many times as adults (iteroparity).[55][56][57]
Predators
A variety of fauna prey upon ostracods in both aquatic and terrestrial environments. An example of predation in the marine environment is the action of certain Cytherocopina in the cuspidariid clams in detecting ostracods with cilia protruding from inhalant structures, thence drawing the ostracod prey in by a violent suction action.[58] Predation from higher animals also occurs; for example, amphibians such as the rough-skinned newt prey upon certain ostracods.[59]
Bioluminescence
Some ostracods, such as Vargula hilgendorfii, have a light organ in which they produce luminescent chemicals.[60] These ostracods are called "blue sand" or "blue tears" and glow blue in the dark. Their bioluminescent properties made them valuable to the Japanese during World War II, when the Japanese army collected large amounts from the ocean to use as a convenient light for reading maps and other papers at night. The light from these ostracods, called umihotaru in Japanese, was sufficient to read by but not bright enough to give away troops' position to enemies.[61] Bioluminescence has evolved twice in ostracods; once in Cypridinidae, and once in Halocyprididae.[62] In bioluminescent Halocyprididae a green light is produced within carapace glands, and in Cypridinidae a blue light is produced and extruded from the upper lip.[63][64] Most species use the light as predation defense, but the male of at least 75 known species of the Cypridinidae, restricted to the Caribbean, use pulses of light to attract females. This bioluminiscent courtship display has only evolved once in ostracods, in a cypridinid group named Luxorina that originated at least 151 million years ago.[65][66] Ostracods with bioluminescent courtship show higher rates of speciation than those who simply use light as protection against predators.[67]
Classification
Class Ostracoda is divided into following living clades:[68]
- Subclass Myodocopa
- Order Myodocopida
- Suborder Myodocopina
- Superfamily Cypridinoidea (1 family)
- Superfamily Cylindroleberidoidea (1 family)
- Superfamily Sarsielloidea (3 families)
- Suborder Myodocopina
- Order Halocyprida
- Suborder Halocypridina
- Superfamily Thaumatocypridoidea (1 family)
- Superfamily Halocypridoidea (1 family)
- Suborder Cladocopina
- Superfamily Cladocopoidea (1 family)
- Suborder Halocypridina
- Order Myodocopida
- Subclass Podocopa
- Order Palaeocopida
- Suborder Kirkbyocopina
- Superfamily Puncioidea (1 family)
- Suborder Kirkbyocopina
- Order Platycopida
- Suborder Platycopina
- Superfamily Cytherelloidea (1 family)
- Suborder Platycopina
- Order Podocopida
- Suborder Cytherocopina
- Superfamily Cytheroidea (27 families)
- Superfamily Terrestricytheroidea (1 family)
- Suborder Cypridocopina
- Superfamily Macrocypridoidea (1 family)
- Superfamily Pontocypridoidea (1 family)
- Superfamily Cypridoidea (4 families)
- Suborder Darwinulocopina
- Superfamily Darwinuloidea (1 family)
- Suborder Bairdiocopina
- Superfamily Bairdioidea (3 families)
- Suborder Sigilliocopina
- Superfamily Sigillioidea (1 family)
- Suborder Cytherocopina
- Order Palaeocopida
See also
- Mari Mari Group, fossil formation in the state of Amazonas of northwestern Brazil
References
- ↑ Williams, Mark; Siveter, David J.; Salas, María José; Vannier, Jean; Popov, Leonid E.; Ghobadi Pour, Mansoureh (2008). "The earliest ostracods: the geological evidence". Senckenbergiana Lethaea 88: 11–21. doi:10.1007/BF03043974. https://link.springer.com/article/10.1007/BF03043974.
- ↑ 2.0 2.1 Brandão, S.N.; Antonietto, L.S; Nery, D.G.; Santos, S.G.; Karanovic, I. (2023). World Ostracoda Database. Accessed at https://www.marinespecies.org/ostracoda on 2023-09-12. doi:10.14284/364
- ↑ Freshwater Life: A field guide to the plants and animals of southern Africa
- ↑ The Triassic-Jurassic Terrestrial Transition: 37
- ↑ Jöst, Anna B. (January 2012). Ecological Evaluation of Ostracode (Crustacea) Occurrence in the City of Munich & Phylogenetic Relationships between Ostracode Species and Populations from Bavaria (master thesis). Ludwig Maximilian University of Munich.
- ↑ Ostracoda - Oxford Academic
- ↑ Richard A. Fortey; Richard H. Thomas (1998). Arthropod Relationships. Chapman & Hall. ISBN 978-0-412-75420-3.
- ↑ S. Yamaguchi; K. Endo (2003). "Molecular phylogeny of Ostracoda (Crustacea) inferred from 18S ribosomal DNA sequences: implication for its origin and diversification". Marine Biology 143 (1): 23–38. doi:10.1007/s00227-003-1062-3.
- ↑ Zaharoff, Alexander K.; Lindgren, Annie R.; Wolfe, Joanna M.; Oakley, Todd H. (2013-01-01). "Phylotranscriptomics to Bring the Understudied into the Fold: Monophyletic Ostracoda, Fossil Placement, and Pancrustacean Phylogeny" (in en). Molecular Biology and Evolution 30 (1): 215–233. doi:10.1093/molbev/mss216. ISSN 0737-4038. PMID 22977117.
- ↑ David J. Siveter; Derek E. G. Briggs; Derek J. Siveter; Mark D. Sutton (2010). "An exceptionally preserved myodocopid ostracod from the Silurian of Herefordshire, UK". Proceedings of the Royal Society B 277 (1687): 1539–1544. doi:10.1098/rspb.2009.2122. PMID 20106847.
- ↑ Malcolm B. Hart (1972). "A correlation of the macrofaunal and microfaunal zonations of the Gault Clay in southeast England". Geological Journal (Special Issue 5): 267–288.
- ↑ Noriyuki Ikeya, Akira Tsukagoshi; David J. Horne (2005). "Preface: The phylogeny, fossil record and ecological diversity of ostracod crustaceans". Hydrobiologia 538 (1–3): vii–xiii. doi:10.1007/s10750-004-4914-z.
- ↑ Oldest penis:
The oldest fossilised penis discovered to date dates back around 100 million years. It belongs to a crustacean called an ostracod, discovered in Brazil and measuring just 1mm across. - ↑ World's oldest sperm 'preserved in bat poo', Anna Salleh, ABC Online Science, 14 May 2014, accessed 15 May 2014
- ↑ Butterflies of the Cambrian benthos? Shield position in bradoriid arthropods - Idunn
- ↑ Embryonic development clarifies polyphyly in ostracod crustaceans
- ↑ Expression of two engrailed genes in the embryo of Vargula hilgendorfii (Müller, 1890) (Ostracoda: Myodocopida)
- ↑ Phylogeny, Ontogeny, and Morphology of Living and Fossil Thaumatocypridacea (Myodocopa: Ostracoda)
- ↑ 19.0 19.1 19.2 19.3 Robert D. Barnes (1982). Invertebrate Zoology. Philadelphia: Holt-Saunders International. pp. 680–683. ISBN 978-0-03-056747-6.
- ↑ Ostracoda - an overview | ScienceDirect Topics
- ↑ Karanovic, Ivana (2012). Recent Freshwater Ostracods of the World: Crustacea, Ostracoda, Podocopida. Springer. pp. 5–47. doi:10.1007/978-3-642-21810-1. ISBN 978-3-642-21809-5. Limited preview via Google Books
- ↑ Oxygen as a Driver of Early Arthropod Micro-Benthos Evolution
- ↑ Corbari, Laure; Carbonel, Pierre; Massabuau, Jean-Charles (2005). "The early life history of tissue oxygenation in crustaceans: the strategy of the myodocopid ostracod Cylindroleberis mariae". Journal of Experimental Biology 208 (4): 661–670. doi:10.1242/jeb.01427. PMID 15695758.
- ↑ Occurrence of hemocyanin in ostracod crustaceans
- ↑ Oakley, Todd H.; Cunningham, Clifford W. (2002). "Molecular phylogenetic evidence for the independent evolutionary origin of an arthropod compound eye". Proceedings of the National Academy of Sciences USA 99 (3): 1426–1430. doi:10.1073/pnas.032483599. PMID 11818548. Bibcode: 2002PNAS...99.1426O.
- ↑ The darkness syndrome in subsurface-shallow and deep-sea dwelling Ostracoda (Crustacea)
- ↑ Tanaka, Gengo (2006). "Functional morphology and light-gathering ability of podocopid ostracod eyes and the palaeontological implications". Zoological Journal of the Linnean Society 147 (1): 97–108. doi:10.1111/j.1096-3642.2006.00216.x.
- ↑ Xing, Lida; Sames, Benjamin; McKellar, Ryan C.; Xi, Dangpeng; Bai, Ming; Wan, Xiaoqiao (2018). "A gigantic marine ostracod (Crustacea: Myodocopa) trapped in mid-Cretaceous Burmese amber". Scientific Reports 8 (1): 1365. doi:10.1038/s41598-018-19877-y. 1365. PMID 29358761. Bibcode: 2018NatSR...8.1365X.
- ↑ Talking about a re-evolution: blind alleys in ostracod phylogeny
- ↑ D. J. Horne (2007). "A mutual temperature range method for European Quaternary non-marine Ostracoda" (PDF). Geophysical Research Abstracts 9: 00093. http://www.cosis.net/abstracts/EGU2007/00093/EGU2007-J-00093.pdf?PHPSESSID=e.
- ↑ Brandão, Simone N.; Hoppema, Mario; Kamenev, Gennady M.; Karanovic, Ivana; Riehl, Torben; Tanaka, Hayato; Vital, Helenice; Yoo, Hyunsu et al. (2019). "Review of Ostracoda (Crustacea) living below the Carbonate Compensation Depth and the deepest record of a calcified ostracod". Progress in Oceanography 178: 102144. doi:10.1016/j.pocean.2019.102144. Bibcode: 2019PrOce.17802144B. https://www.sciencedirect.com/science/article/abs/pii/S0079661119300655.
- ↑ Zooplankton and Micronekton of the North Sea 2.0 – Ordo Platycopida
- ↑ Key Events in the Ecological Radiation of the Ostracoda
- ↑ "News from mid-Cretaceous 'Burmese Amber'" (in de). https://fgga.univie.ac.at/news/news-views/detailansicht/news/news-from-mid-cretaceous-burmese-amber-1/.
- ↑ Thorp and Covich's Freshwater Invertebrates: Ecology and General Biology
- ↑ Lost Sex
- ↑ Seed Shrimp, Mussel Shrimp (Freshwater Ostracods) scientific name: (Crustacea: Ostracoda: Podocopa)
- ↑ The Genome Sizes of Ostracod Crustaceans Correlate with Body Size and Evolutionary History, but not Environment
- ↑ Thorp and Covich's Freshwater Invertebrates, Volume 5: Keys to Neotropical and Antarctic Fauna
- ↑ Karanovic, I.; Eberhard, S.; Perina, G. (2012). "Austromesocypris bluffensis sp. n. (Crustacea, Ostracoda, Cypridoidea, Scottiinae) from subterranean aquatic habitats in Tasmania, with a key to world species of the subfamily". ZooKeys (215): 1–31. doi:10.3897/zookeys.215.2987. PMID 22936868.
- ↑ J. D. Stout (1963). "The Terrestrial Plankton". Tuatara 11 (2): 57–65. https://nzetc.victoria.ac.nz/tm/scholarly/tei-Bio11Tuat02-t1-body-d1.html.
- ↑ K. Martens; I. Schon; C. Meisch; D. J. Horne (2008). "Global diversity of ostracods (Ostracoda, Crustacea) in freshwater". Hydrobiologia 595 (1): 185–193. doi:10.1007/s10750-007-9245-4.
- ↑ 43.0 43.1 K. Martens, S. A. Halse & I. Schon (2012). "Nine new species of Bennelongia De Deckker & McKenzie, 1981 (Crustacea, Ostracoda) from Western Australia, with the description of a new subfamily". European Journal of Taxonomy 8: 1–56. http://www.europeanjournaloftaxonomy.eu/index.php/ejt/article/view/98.
- ↑ Iepure, Sanda; Wysocka, Anna; Sarbu, Serban M.; Kijowska, Michalina; Namiotko, Tadeusz (2023). "A new extremophile ostracod crustacean from the Movile Cave sulfidic chemoautotrophic ecosystem in Romania". Scientific Reports 13 (1): 6112. doi:10.1038/s41598-023-32573-w. PMID 37059813. Bibcode: 2023NatSR..13.6112I.
- ↑ Mercado-Salas, Nancy F.; Khodami, Sahar; Martínez Arbizu, Pedro (2021). "Copepods and ostracods associated with bromeliads in the Yucatán Peninsula, Mexico". PLOS ONE 16 (3): e0248863. doi:10.1371/journal.pone.0248863. PMID 33735283. Bibcode: 2021PLoSO..1648863M.
- ↑ A new extremophile ostracod crustacean from the Movile Cave sulfidic chemoautotrophic ecosystem in Romania
- ↑ Horne, D. J.; Martens, Koen (1998). "An assessment of the importance of resting eggs for the evolutionary success of non-marine Ostracoda (Crustacea)". Evolutionary and ecological aspects of crustacean diapause. 52. Advances in Limnology. pp. 549–561. ISBN 9783510470549. http://www.schweizerbart.de/publications/detail/isbn/9783510470549/Evolutionary_and_ecological_aspects_of_crustacean_diapause.
- ↑ The ontogeny of two species of Darwinuloidea (Ostracoda, Crustacea)
- ↑ Smith RJ, Kamiya T, Horne DJ. Living males of the 'ancient asexual' Darwinulidae (Ostracoda: Crustacea). Proc Biol Sci. 2006 Jun 22;273(1593):1569-78. doi: 10.1098/rspb.2005.3452. PMID: 16777754; PMCID: PMC1560310
- ↑ Field Guide to Freshwater Invertebrates of North America
- ↑ Recent Freshwater Ostracods of the World: Crustacea, Ostracoda, Podocopida
- ↑ Specieswatch: ancient crustaceans still going strong after 450m years
- ↑ The Light and Smith Manual : Intertidal Invertebrates from Central California to Oregon – Page 419
- ↑ Recent Freshwater Ostracods of the World: Crustacea, Ostracoda, Podocopida
- ↑ Strategies of crustacean growth - Australian Museum
- ↑ Crustacean Issues 3: Factors in Adult Growth
- ↑ Evolution and Phylogeny of Pancrustacea: A Story of Scientific Method
- ↑ John D. Gage; Paul A. Tyler (1992-09-28). Deep-Sea Biology: A Natural History of Organisms at the Deep-Sea Floor. University of Southampton. ISBN 978-0-521-33665-9.
- ↑ C. Michael Hogan (2008). "Rough-skinned Newt ("Taricha granulosa")". http://www.globaltwitcher.com/artspec_information.asp?thingid=43182.
- ↑ Osamu Shimomura (2006). "The ostracod Cypridina (Vargula) and other luminous crustaceans". Bioluminescence: Chemical Principles and Methods. World Scientific. pp. 47–89. ISBN 978-981-256-801-4. https://books.google.com/books?id=DNrTfH5PcWoC&pg=PA49.
- ↑ Jabr, Ferris. "The Secret History of Bioluminescence". https://www.hakaimagazine.com/article-long/secret-history-bioluminescence.
- ↑ Collecting and processing marine ostracods - Oxford Academic
- ↑ Sexual Morphology, Reproduction and the Evolution of Bioluminescence in Ostracoda
- ↑ Two New Bioluminescent Ostracode Genera, Enewton And Photeros (Myodocopida: Cypridinidae), with Three New Species from Jamaica
- ↑ Sexual Signals Persist over Deep Time: Ancient Co-option of Bioluminescence for Courtship Displays in Cypridinid Ostracods
- ↑ Female ostracods respond to and intercept artificial conspecific male luminescent courtship displays
- ↑ Bioluminescence Is Nature's Love Light
- ↑ Ostracods - what are they? - Lake Biwa Museum
External links
- Kempf Database Ostracoda
- Ostracoda fact sheet, Guide to the Marine Zooplankton of South-eastern Australia]
- Key to the two subclasses
- International Research Group on Ostracoda
- Ostracoda Fact Sheet
- Huge sperm of ancient crustaceans
- World Ostracoda Database
Wikidata ☰ Q276412 entry
 |



