Biology:mir-133 microRNA precursor family
| mir-133 microRNA precursor family | |
|---|---|
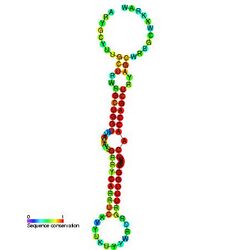 Predicted secondary structure and sequence conservation of mir-133 | |
| Identifiers | |
| Symbol | mir-133 |
| Rfam | RF00446 |
| miRBase | MI0000450 |
| miRBase family | MIPF0000029 |
| Other data | |
| RNA type | Gene; miRNA |
| Domain(s) | Eukaryota |
| GO | 0035195 0035068 |
| SO | 0001244 |
| PDB structures | PDBe |
mir-133 is a type of non-coding RNA called a microRNA that was first experimentally characterised in mice.[1] Homologues have since been discovered in several other species including invertebrates such as the fruitfly Drosophila melanogaster. Each species often encodes multiple microRNAs with identical or similar mature sequence. For example, in the human genome there are three known miR-133 genes: miR-133a-1, miR-133a-2 and miR-133b found on chromosomes 18, 20 and 6 respectively. The mature sequence is excised from the 3' arm of the hairpin. miR-133 is expressed in muscle tissue and appears to repress the expression of non-muscle genes.[2]
Regulation
It is proposed that Insulin activates the translocation of SREBP-1c (BHLH) active form from the endoplasmic reticulum (ER) to the nucleus and, concomitantly, induces SREPB-1c expression via PI3K signaling pathway. SREBP-1c mediates MEF2C downregulation through a mechanism that remains to be determined. As a consequence of lower MEF2C binding on their enhancer region, the transcription of miR-1 and miR-133a is reduced, leading to decreased levels of their mature forms in muscle, after insulin treatment. Altered activation of PI3K and SREBP-1c may explain the defective regulation of miR-1 and miR-133a expression in response to insulin in muscle of type 2 diabetic patients.[3]
Targets of miR-133
microRNAs act by lowering the expression of genes by binding to target sites in the 3' UTR of the mRNAs. Luo et al.. demonstrated that the HCN2 K+ channel gene contains a target of miR-133.[4] Yin et al.. showed that the Mps1 kinase gene in zebrafish is a target.[5] Boutz et al.. showed that nPTB (neuronal polypyrimidine tract-binding protein) is a target and likely contains two target sites for miR-133.[6] Xiao et al.. show that ether-a-go-go related gene (ERG) a K+ channel is a target of miR-133.[7]
miR-133 directly and negatively regulates NFATc4.[8][9]
RhoA expression is negatively regulated by miR-133a in bronchial smooth muscles (BSM)and miR-133a downregulation causes an upregulation of RhoA, resulting in an augmentation of contraction and BSM hyperresponsiveness.[10]
BMP2 downregulates multiple mIRs, of which one, miR-133, directly inhibits Runx2, an early BMP response gene essential for bone formation. Although miR-133 is known to promote MEF-2-dependent myogenesis, it also inhibits Runx2-mediated osteogenesis. BMP2 controls bone cell determination by inducing miRNAs that target muscle genes but mainly by down-regulating multiple miRNAs that constitute an osteogenic program, thereby releasing from inhibition pathway components required for cell lineage commitment establish a mechanism for BMP morphogens to selectively induce a tissue-specific phenotype and suppress alternative lineages.[11]
Nicotine activates α7-nAChR and downregulates the levels of miR-133 and miR-590 leading to significant upregulation of expression of TGF-β1 and TGF-βRII at the protein level establishing miR-133 and miR-590 as repressors of TGF-β1 and TGF-βRII.[12]
miR-133 enhances myoblast proliferation by repressing serum response factor (SRF)[13]
mIR-133 suppresses SP1 expression[14]
In rats, miR-133b is expressed in retinal dopaminergicamacrine cell, and this expression is significantly increased during early stage during retinal degeneration. This overexpression leads to downregulation of the transcription factor PITX3.[15] miR-133a is down regulated in diabetic cardiomyopathy.[16]
miR-133 suppresses Prdm16 expression in skeletal muscle stem cells (satellite cells), which controls myogenic vs. brown adipogenic lineage determination in these cells.[17]
References
- ↑ "Identification of tissue-specific microRNAs from mouse". Current Biology 12 (9): 735–9. Apr 2002. doi:10.1016/S0960-9822(02)00809-6. PMID 12007417.
- ↑ "MicroRNA regulation of cell lineages in mouse and human embryonic stem cells". Cell Stem Cell 2 (3): 219–29. Mar 2008. doi:10.1016/j.stem.2008.01.016. PMID 18371447.
- ↑ "The microRNA signature in response to insulin reveals its implication in the transcriptional action of insulin in human skeletal muscle and the role of a sterol regulatory element-binding protein-1c/myocyte enhancer factor 2C pathway". Diabetes 58 (11): 2555–64. Nov 2009. doi:10.2337/db09-0165. PMID 19720801.
- ↑ "Down-regulation of miR-1/miR-133 contributes to re-expression of pacemaker channel genes HCN2 and HCN4 in hypertrophic heart". The Journal of Biological Chemistry 283 (29): 20045–52. Jul 2008. doi:10.1074/jbc.M801035200. PMID 18458081.
- ↑ "Fgf-dependent depletion of microRNA-133 promotes appendage regeneration in zebrafish". Genes & Development 22 (6): 728–33. Mar 2008. doi:10.1101/gad.1641808. PMID 18347091.
- ↑ "MicroRNAs regulate the expression of the alternative splicing factor nPTB during muscle development". Genes & Development 21 (1): 71–84. Jan 2007. doi:10.1101/gad.1500707. PMID 17210790.
- ↑ "MicroRNA miR-133 represses HERG K+ channel expression contributing to QT prolongation in diabetic hearts". The Journal of Biological Chemistry 282 (17): 12363–7. Apr 2007. doi:10.1074/jbc.C700015200. PMID 17344217.
- ↑ "NFATc4 is negatively regulated in miR-133a-mediated cardiomyocyte hypertrophic repression". American Journal of Physiology. Heart and Circulatory Physiology 298 (5): H1340-7. May 2010. doi:10.1152/ajpheart.00592.2009. PMID 20173049.
- ↑ "Reciprocal repression between microRNA-133 and calcineurin regulates cardiac hypertrophy: a novel mechanism for progressive cardiac hypertrophy". Hypertension 55 (4): 946–52. Apr 2010. doi:10.1161/HYPERTENSIONAHA.109.139519. PMID 20177001.
- ↑ "MicroRNAs and their therapeutic potential for human diseases: MiR-133a and bronchial smooth muscle hyperresponsiveness in asthma". Journal of Pharmacological Sciences 114 (3): 264–8. 2010. doi:10.1254/jphs.10R10FM. PMID 20953121.
- ↑ "A microRNA signature for a BMP2-induced osteoblast lineage commitment program". Proceedings of the National Academy of Sciences of the United States of America 105 (37): 13906–11. Sep 2008. doi:10.1073/pnas.0804438105. PMID 18784367.
- ↑ "Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines". Cardiovasc. Res. 83 (3): 465–72. 2009. doi:10.1093/cvr/cvp130. PMID 19398468.
- ↑ "The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation". Nat. Genet. 38 (2): 228–33. 2006. doi:10.1038/ng1725. PMID 16380711.
- ↑ "MicroRNA-133 Controls Vascular Smooth Muscle Cell Phenotypic Switch In Vitro and Vascular Remodeling In Vivo". Circulation Research 109 (8): 880–893. 2011. doi:10.1161/CIRCRESAHA.111.240150. PMID 21852550.
- ↑ "A microRNA, mir133b, suppresses melanopsin expression mediated by failure dopaminergic amacrine cells in RCS rats". Cellular Signalling 24 (3): 685–98. Mar 2012. doi:10.1016/j.cellsig.2011.10.017. PMID 22101014.
- ↑ "Differential expression of dicer, miRNAs, and inflammatory markers in diabetic Ins2+/- Akita hearts". Cell Biochem. Biophys. 68 (1): 25–35. 2014. doi:10.1007/s12013-013-9679-4. PMID 23797610.
- ↑ "MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16". Cell Metabolism 17 (2): 210–24. Feb 2013. doi:10.1016/j.cmet.2013.01.004. PMID 23395168.
Further reading
- "Relation of circulating MicroRNA-133a concentrations with myocardial damage and clinical prognosis in ST-elevation myocardial infarction". American Heart Journal 164 (5): 706–14. Nov 2012. doi:10.1016/j.ahj.2012.08.004. PMID 23137501.
External links
- Page for mir-133 microRNA precursor family at Rfam
- miRBase entry for mir-133
- MicroRNA of the week at the miRNA blog
 |

