Biology:Mechanism of autism
The mechanisms of autism are the molecular and cellular processes believed to cause or contribute to the symptoms of autism. Multiple processes are hypothesized to explain different autism spectrum features. These hypotheses include defects in synapse structure and function,[1][2] reduced synaptic plasticity,[3] disrupted neural circuit function, gut–brain axis dyshomeostasis,[4][5][6] neuroinflammation,[7] and altered brain structure or connectivity.[8][9][10][11]
Pathophysiology
Unlike some brain disorders which have clear molecular hallmarks that can be observed in every affected individual, such as Alzheimer's disease or Parkinson's disease, autism does not have a unifying mechanism at the molecular, cellular, or systems level. The autism spectrum may comprise a small set of disorders that converge on a few common molecular pathways, or it may be a large set of disorders with diverse mechanisms.[13] Autism appears to result from developmental factors that affect many or all functional brain systems.[14] Some factors may disturb the timing of brain development rather than the final product.[12]
Brain growth
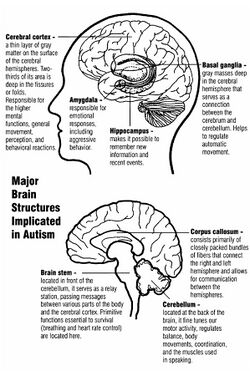
Neuroanatomical studies and the association between autism and teratogens strongly suggest that autism affects brain development soon after conception.[15] This anomaly appears to start a cascade of pathological events in the brain that are significantly influenced by environmental factors.[16] Just after birth, the brains of children with autism tend to grow faster than usual, followed by normal or relatively slower growth in childhood.[17] It is unknown whether early brain overgrowth occurs in all children with autism. It appears to be most prominent in the frontal and temporal lobes, which are associated with higher cognitive specializations such as social cognition, and language development.[18] Hypotheses for the cellular and molecular bases of pathological early overgrowth include an excess of neurons that causes local overconnectivity in key brain regions,[17] and disturbed neuronal migration during early gestation.[19][20]
Synapse dysfunction
Synapse and dendritic spine growth may be disrupted in autism due to impaired neurexin–neuroligin cell-adhesion signaling[21] or dysregulated synthesis of synaptic proteins.[22][23] Disrupted synaptic development may also contribute to epilepsy, which may explain why the two conditions are associated.[24]Studies have suggested that excitatory–inhibitory networks may be imbalanced in autism.[20]
Neurotransmitters such as serotonin, dopamine, and glutamate have been implicated in autism.[1] Fragile X, the most common genetic cause of autism, is linked to dysfunction of group I metabotropic glutamate receptors (mGluR), leading some to consider their potential role in autism.[25]
Altered circuit connectivity

The underconnectivity theory of autism posits that autistic people tend to have fewer high-level neural connections and less global synchronization, along with an excess of low-level processes.[27] Functional connectivity studies have found both hypo- and hyperconnectivity in brains of autistic people.[28] Hypoconnectivity is commonly observed for interhemispheric and cortico-cortical functional connectivity.[29] Some studies have found local overconnectivity in the cerebral cortex and weak functional connections between the frontal lobe and the rest of the cortex.[30] Abnormal default mode network (task-negative) connectivity is often observed. Toggling between task-negative network activation and task-positive network activation (consisting of the dorsal attention network and salience network) may be less efficient, possibly reflecting a disturbance of self-referential thought.[31] Such patterns of low function and aberrant activation in the brain may depend on whether the brain is performing social or nonsocial tasks.[32]
Some studies have suggested that autism is a disorder of the association cortex.[33] Event-related potentials with respect to attention, orientation to auditory and visual stimuli, novelty detection, language and face processing, and information storage are altered in autistic individuals; several studies have found a preference for nonsocial stimuli.[34] Magnetoencephalography studies have observed delayed processing of auditory signals in autistic children.[35]
The mirror neuron system (MNS) theory of autism hypothesizes that disrupted development of the MNS impairs autistic people's ability to imitate others, leading to core autistic features of social impairment and communication difficulties. In animals, the MNS activates when an animal performs an action or observes another animal perform the same action. The MNS may contribute to an individual's understanding of other people by enabling the modeling of their behavior via embodied simulation of their actions, intentions, and emotions.[36] [37] Several studies have tested this hypothesis by demonstrating structural abnormalities in MNS regions of individuals with ASD, delay in the activation in the core circuit for imitation in individuals with ASD, and a correlation between reduced MNS activity and severity of the syndrome in children with ASD.[38] However, individuals with autism also have abnormal brain activation in many circuits outside the MNS[39] and the MNS theory does not explain the normal performance of children with autism on imitation tasks that involve a goal or object.[40]
Common copy number variation associations have suggested similarities between the mechanisms of autism and schizophrenia. For loci such as 16p11.2, 16p13.1, 22p11, and 22q13, deletion is associated with autism whereas duplication is associated with schizophrenia. Conversely, 1q21.1 and 22p11.2 duplication is associated with autism and deletion with schizophrenia.[41]
Inflammation
The immune system is thought to play an important role in autism. Children with autism have been found by researchers to have inflammation of both the peripheral and central immune systems as indicated by increased levels of pro-inflammatory cytokines and significant activation of microglia.[42][43][7] Biomarkers of abnormal immune function have also been associated with increased impairments in behaviors that are characteristic of the core features of autism such as, deficits in social interactions and communication.[43] Interactions between the immune system and the nervous system begin early during the embryonic stage of life, and successful neurodevelopment depends on a balanced immune response. It is thought that activation of a pregnant mother's immune system such as from environmental toxicants or infection can contribute to causing autism through causing a disruption of brain development.[44][45][46] This is supported by recent studies that have found that infection during pregnancy is associated with an increased risk of autism.[47][48]
Some evidence suggests that gut–brain axis abnormalities may be involved by means of impaired serotonin signaling and inflammation.[6] A 2015 review proposed that immune dysregulation, gastrointestinal inflammation, autonomic nervous system malfunction, gut microbiota alterations, and food metabolites may cause brain neuroinflammation and dysfunction.[4] A 2016 review concluded that enteric nervous system abnormalities might play a role in neurological disorders such as autism.[5]
Metabolism
Some data suggests neuronal overgrowth observed in autism may be caused by an increase in several growth hormones[49] or impaired regulation of growth factor receptors. Some inborn errors of metabolism are associated with autism, but probably account for less than 5% of cases.[50]
Neuropsychology
Two major categories of cognitive theories have been proposed to explain links between autistic brains and behavior.
Social cognition
The first category focuses on deficits in social cognition. Simon Baron-Cohen's empathizing–systemizing theory postulates that autistic individuals can systemize—that is, they can develop internal rules of operation to handle events inside the brain—but are less effective at empathizing by handling events generated by other agents. An extension, the extreme male brain theory, hypothesizes that autism is an extreme case of the male brain, defined psychometrically as individuals in whom systemizing is better than empathizing.[51] These theories are somewhat related to Baron-Cohen's earlier theory of mind approach, which hypothesizes that autistic behavior arises from an inability to ascribe mental states to oneself and others. The theory of mind hypothesis is supported by the atypical responses of children with autism to the Sally–Anne test for reasoning about others' motivations,[51] and the mirror neuron system theory of autism described in Pathophysiology maps well to the hypothesis.[38] However, most studies have found no evidence of impairment in autistic individuals' ability to understand other people's basic intentions or goals; instead, data suggests that impairments are found in understanding more complex social emotions or in considering others' viewpoints.[52]
Nonsocial cognition
The second category focuses on nonsocial or general processing: the executive functions such as working memory, planning, inhibition. In his review, Kenworthy states that "the claim of executive dysfunction as a causal factor in autism is controversial", however, "it is clear that executive dysfunction plays a role in the social and cognitive deficits observed in individuals with autism".[53] Tests of core executive processes such as eye movement tasks indicate improvement from late childhood to adolescence, but performance never reaches typical adult levels.[54] A strength of the theory is predicting stereotyped behavior and narrow interests;[55] two weaknesses are that executive function is hard to measure[53] and that executive function deficits have not been found in young children with autism.[56]
Weak central coherence theory
Weak central coherence theory hypothesizes that a limited ability to see the big picture underlies the central disturbance in autism. One strength of this theory is predicting special talents and peaks in performance in autistic people.[57] A related theory—enhanced perceptual functioning—focuses more on the superiority of locally oriented and perceptual operations in autistic individuals.[58] Yet another, monotropism, posits that autism stems from a different cognitive style, tending to focus attention (or processing resources) intensely, to the exclusion of other stimuli.[59] These theories map well from the underconnectivity theory of autism.
Issues with categories
Neither category is satisfactory on its own; social cognition theories poorly address autism's rigid and repetitive behaviors, while most of the nonsocial theories have difficulty explaining social impairment and communication difficulties.[60] A combined theory based on multiple deficits may prove to be more useful.[61]
References
- ↑ 1.0 1.1 "Autism". Lancet 374 (9701): 1627–38. 2009. doi:10.1016/S0140-6736(09)61376-3. PMID 19819542.
- ↑ "The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders". Trends Neurosci 32 (7): 402–12. 2009. doi:10.1016/j.tins.2009.04.003. PMID 19541375. PMC 10354373. http://www.hal.inserm.fr/inserm-00401195/en/.
- ↑ "Autism and brain development". Cell 135 (3): 396–400. 2008. doi:10.1016/j.cell.2008.10.015. PMID 18984148.
- ↑ 4.0 4.1 "Gastrointestinal symptoms and autism spectrum disorder: links and risks - a possible new overlap syndrome.". Pediatric Health Med Ther 6: 153–166. 2015. doi:10.2147/PHMT.S85717. PMID 29388597.
- ↑ 5.0 5.1 "The bowel and beyond: the enteric nervous system in neurological disorders". Nat Rev Gastroenterol Hepatol 13 (9): 517–28. September 2016. doi:10.1038/nrgastro.2016.107. PMID 27435372.
- ↑ 6.0 6.1 "Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders.". Pharmacol Res 132: 1–6. 2018. doi:10.1016/j.phrs.2018.03.020. PMID 29614380.
- ↑ 7.0 7.1 "Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism". Frontiers in Physiology 5: 150. 2014. doi:10.3389/fphys.2014.00150. PMID 24795645.
- ↑ "A Unifying Theory for Autism: The Pathogenetic Triad as a Theoretical Framework". Frontiers in Psychiatry 12: 767075. November 2021. doi:10.3389/fpsyt.2021.767075. PMID 34867553.
- ↑ "Neurobiological correlates of autism: a review of recent research". Child Neuropsychol 12 (1): 57–79. 2006. doi:10.1080/09297040500253546. PMID 16484102.
- ↑ "The role of the neurobiologist in redefining the diagnosis of autism". Brain Pathol 17 (4): 408–11. 2007. doi:10.1111/j.1750-3639.2007.00103.x. PMID 17919126.
- ↑ "Diagnosis of autism". BMJ 327 (7413): 488–93. 2003. doi:10.1136/bmj.327.7413.488. PMID 12946972.
- ↑ 12.0 12.1 "Neuroanatomy of autism". Trends Neurosci 31 (3): 137–45. 2008. doi:10.1016/j.tins.2007.12.005. PMID 18258309.
- ↑ "Autism: many genes, common pathways?". Cell 135 (3): 391–95. 2008. doi:10.1016/j.cell.2008.10.016. PMID 18984147.
- ↑ "The study of autism as a distributed disorder". Ment Retard Dev Disabil Res Rev 13 (1): 85–95. 2007. doi:10.1002/mrdd.20141. PMID 17326118.
- ↑ "The teratology of autism". Int J Dev Neurosci 23 (2–3): 189–99. 2005. doi:10.1016/j.ijdevneu.2004.11.001. PMID 15749245.
- ↑ "The neuropathology of autism". Brain Pathol 17 (4): 422–33. 2007. doi:10.1111/j.1750-3639.2007.00100.x. PMID 17919128. PMC 8095561. https://www.academia.edu/14210559.
- ↑ 17.0 17.1 "Mapping early brain development in autism". Neuron 56 (2): 399–413. 2007. doi:10.1016/j.neuron.2007.10.016. PMID 17964254.
- ↑ "Advances in autism". Annu Rev Med 60: 367–80. 2009. doi:10.1146/annurev.med.60.053107.121225. PMID 19630577.
- ↑ "The neuropathology of autism: where do we stand?". Neuropathol Appl Neurobiol 34 (1): 4–11. 2008. doi:10.1111/j.1365-2990.2007.00872.x. PMID 17971078.
- ↑ 20.0 20.1 "Searching for ways out of the autism maze: genetic, epigenetic and environmental clues". Trends Neurosci 29 (7): 349–58. 2006. doi:10.1016/j.tins.2006.05.010. PMID 16808981.
- ↑ "Neuroligins and neurexins link synaptic function to cognitive disease". Nature 455 (7215): 903–11. 2008. doi:10.1038/nature07456. PMID 18923512. Bibcode: 2008Natur.455..903S.
- ↑ "The autistic neuron: troubled translation?". Cell 135 (3): 401–06. 2008. doi:10.1016/j.cell.2008.10.017. PMID 18984149.
- ↑ "Fragile X: translation in action". Neuropsychopharmacology 33 (1): 84–7. 2008. doi:10.1038/sj.npp.1301610. PMID 17940551.
- ↑ "Convulsing toward the pathophysiology of autism". Brain Dev 31 (2): 95–103. 2009. doi:10.1016/j.braindev.2008.09.009. PMID 19006654.
- ↑ "Correction of fragile X syndrome in mice". Neuron 56 (6): 955–62. 2007. doi:10.1016/j.neuron.2007.12.001. PMID 18093519.
- ↑ "Opening a window to the autistic brain". PLOS Biol 2 (8): E267. 2004. doi:10.1371/journal.pbio.0020267. PMID 15314667.
- ↑ "Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry". Cereb Cortex 17 (4): 951–61. 2007. doi:10.1093/cercor/bhl006. PMID 16772313.
- ↑ "Neuropsychologic functioning in children with autism: further evidence for disordered complex information-processing". Child Neuropsychol 12 (4–5): 279–98. 2006. doi:10.1080/09297040600681190. PMID 16911973.
- ↑ "Characteristics of Brains in Autism Spectrum Disorder: Structure, Function and Connectivity across the Lifespan". Exp Neurobiol 24 (4): 273–84. December 2015. doi:10.5607/en.2015.24.4.273. PMID 26713076.
- ↑ "Resting state cortical connectivity reflected in EEG coherence in individuals with autism". Biol Psychiatry 62 (3): 270–73. 2007. doi:10.1016/j.biopsych.2006.11.012. PMID 17336944.
- ↑ "Default-mode brain dysfunction in mental disorders: a systematic review". Neurosci Biobehav Rev 33 (3): 279–96. 2009. doi:10.1016/j.neubiorev.2008.09.002. PMID 18824195. https://www.academia.edu/7779107.
- ↑ "Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis". Biol Psychiatry 65 (1): 63–74. 2009. doi:10.1016/j.biopsych.2008.09.022. PMID 18996505.
- ↑ "The new neurobiology of autism: cortex, connectivity, and neuronal organization". Arch Neurol 64 (7): 945–50. 2007. doi:10.1001/archneur.64.7.945. PMID 17620483.
- ↑ "Event related potentials in the understanding of autism spectrum disorders: an analytical review". J Autism Dev Disord 39 (3): 495–510. 2009. doi:10.1007/s10803-008-0652-9. PMID 18850262.
- ↑ "Electrophysiological signatures: magnetoencephalographic studies of the neural correlates of language impairment in autism spectrum disorders". Int J Psychophysiol 68 (2): 149–60. 2008. doi:10.1016/j.ijpsycho.2008.01.012. PMID 18336941.
- ↑ "Self–other relations in social development and autism: multiple roles for mirror neurons and other brain bases". Autism Res 1 (2): 73–90. 2008. doi:10.1002/aur.15. PMID 19360654.
- ↑ "A mirror up to nature". Curr Biol 18 (1): R13–18. 2008. doi:10.1016/j.cub.2007.11.004. PMID 18177704.
- ↑ 38.0 38.1 "The mirror neuron system and the consequences of its dysfunction". Nature Reviews Neuroscience 7 (12): 942–51. 2006. doi:10.1038/nrn2024. PMID 17115076. https://www.academia.edu/13626914.
- ↑ "Development and neurophysiology of mentalizing". Philosophical Transactions of the Royal Society B 358 (1431): 459–73. 2003. doi:10.1098/rstb.2002.1218. PMID 12689373.
- ↑ "Emulation and mimicry for social interaction: a theoretical approach to imitation in autism". Q J Exp Psychol 61 (1): 101–15. 2008. doi:10.1080/17470210701508798. PMID 18038342. https://www.academia.edu/906774.
- ↑ "Evolution in health and medicine Sackler colloquium: Comparative genomics of autism and schizophrenia". Proceedings of the National Academy of Sciences of the United States of America 107 (Suppl 1): 1736–41. 2010. doi:10.1073/pnas.0906080106. PMID 19955444. Bibcode: 2010PNAS..107.1736C.
- ↑ "Immune Dysregulation in Autism Spectrum Disorder". Neurobiology of Autism. International Review of Neurobiology. 113. 2013. pp. 269–302. doi:10.1016/B978-0-12-418700-9.00009-5. ISBN 9780124187009.
- ↑ 43.0 43.1 "The role of immune dysfunction in the pathophysiology of autism". Brain, Behavior, and Immunity 26 (3): 383–92. August 2011. doi:10.1016/j.bbi.2011.08.007. PMID 21906670.
- ↑ "Maternal infection and immune involvement in autism". Trends in Molecular Medicine 17 (7): 389–94. July 2011. doi:10.1016/j.molmed.2011.03.001. PMID 21482187.
- ↑ "Autism risk factors: genes, environment, and gene-environment interactions". Dialogues Clin Neurosci 14 (3): 281–92. 2012. doi:10.31887/DCNS.2012.14.3/pchaste. PMID 23226953.
- ↑ "The immune response in autism: a new frontier for autism research". J Leukoc Biol 80 (1): 1–15. 2006. doi:10.1189/jlb.1205707. PMID 16698940. http://www.jleukbio.org/cgi/content/full/80/1/1.
- ↑ "Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders". Brain, Behavior, and Immunity 44: 100–105. September 2014. doi:10.1016/j.bbi.2014.09.001. PMID 25218900.
- ↑ "Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders". Journal of Autism and Developmental Disorders 40 (12): 1423–30. December 2010. doi:10.1007/s10803-010-1006-y. PMID 20414802.
- ↑ "Update on autism: A review of 1300 reports published in 2008". Epilepsy Behav 16 (4): 569–89. 2009. doi:10.1016/j.yebeh.2009.09.023. PMID 19896907.
- ↑ "Autism and metabolic diseases". J Child Neurol 23 (3): 307–14. 2008. doi:10.1177/0883073807308698. PMID 18079313.
- ↑ 51.0 51.1 "Autism: the empathizing–systemizing (E-S) theory". Annals of the New York Academy of Sciences 1156 (1): 68–80. 2009. doi:10.1111/j.1749-6632.2009.04467.x. PMID 19338503. Bibcode: 2009NYASA1156...68B. http://docs.autismresearchcentre.com/papers/2009_BC_nyas.pdf.
- ↑ "Goals, intentions and mental states: challenges for theories of autism". J Child Psychol Psychiatry 50 (8): 881–92. 2009. doi:10.1111/j.1469-7610.2009.02098.x. PMID 19508497.
- ↑ 53.0 53.1 "Understanding executive control in autism spectrum disorders in the lab and in the real world". Neuropsychol Rev 18 (4): 320–38. 2008. doi:10.1007/s11065-008-9077-7. PMID 18956239.
- ↑ "Neurodevelopment and executive function in autism". Dev Psychopathol 20 (4): 1103–32. 2008. doi:10.1017/S0954579408000527. PMID 18838033.
- ↑ "Executive dysfunction in autism". Trends Cogn Sci 8 (1): 26–32. 2004. doi:10.1016/j.dr.2004.01.001. PMID 14697400. https://www.academia.edu/19291332.
- ↑ "Autism from developmental and neuropsychological perspectives". Annual Review of Clinical Psychology 2: 327–55. 2006. doi:10.1146/annurev.clinpsy.2.022305.095210. PMID 17716073.
- ↑ "The weak coherence account: detail-focused cognitive style in autism spectrum disorders". Journal of Autism and Developmental Disorders 36 (1): 5–25. January 2006. doi:10.1007/s10803-005-0039-0. PMID 16450045. https://www.academia.edu/1754416.
- ↑ "Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception". Journal of Autism and Developmental Disorders 36 (1): 27–43. January 2006. doi:10.1007/s10803-005-0040-7. PMID 16453071.
- ↑ "Attention, monotropism and the diagnostic criteria for autism". Autism 9 (2): 139–56. May 2005. doi:10.1177/1362361305051398. PMID 15857859. http://www.autismusundcomputer.de/english//139.pdf. Retrieved 18 March 2018.
- ↑ "Time to give up on a single explanation for autism". Nature Neuroscience 9 (10): 1218–20. 2006. doi:10.1038/nn1770. PMID 17001340.
- ↑ "Cognitive theories of autism". Dev Rev 27 (2): 224–60. 2007. doi:10.1016/j.dr.2007.02.001. https://strathprints.strath.ac.uk/5154/7/strathprints008124.pdf.
 |