Biology:ATP-binding motif
An ATP-binding motif is a 250-residue sequence within an ATP-binding protein’s primary structure. The binding motif is associated with a protein’s structure and/or function.[1] ATP is a molecule of energy, and can be a coenzyme, involved in a number of biological reactions. ATP is proficient at interacting with other molecules through a binding site. The ATP binding site is the environment in which ATP catalytically actives the enzyme and, as a result, is hydrolyzed to ADP.[2] The binding of ATP causes a conformational change to the enzyme it is interacting with.[3]
The genetic and functional similarity of such a motif demonstrates micro-evolution: proteins have co-opted the same binding sequence from other enzymes rather than developing them independently.[4]
ATP binding sites, which may be representative of an ATP binding motif, are present in many proteins which require an input of energy (from ATP), such sites as active membrane transporters, microtubule subunits, flagellum proteins, and various hydrolytic and proteolytic enzymes.[5]
Primary sequence
The short motifs involving ATP-binding are the Walker motifs, Walker A, also known as the P-loop, and Walker B, as well as the C motif and switch motif.[6]
Walker A motif
The Walker site A has a primary amino acid sequence of Template:Monospace or Template:Monospace. The letter x can represent any amino acid.[7]
Walker B motif
The primary amino acid sequence of the Walker B site is hhhhD, in which h represents any hydrophobic amino acid.[7]
C motif
The C motif, also known as the signature motif, LSGGQ motif, or the linker peptide, has a primary amino acid sequence of LSGGQQ/R/KQR.[8][9]
Due to the variety of different amino acids that can be used in the primary sequence, of both the Walker site A and B, the non-variant amino acids within the sequence are highly conserved. A mutation of any of these amino acids will affect the binding ATP or interfere with the catalytic activity of the enzyme.[7] The primary amino acid sequence determines the three dimensional structure of each motif.[3]
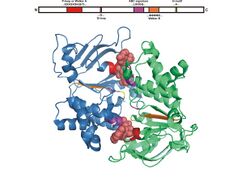
Structure
All of the ATP binding domains are made up of an estimated 250 residues and two subunits, creating a dimer. These residues are folded into six α-helices and five β-strands.[7][9]
Walker A motif
Structurally, the Walker A motif consists of an α-helix and is always followed by a glycine-rich loop.[7]
Walker B motif
The Walker B motif is a β-strand. The Walker motifs are connected to each other by a peptide sequence of about 100 residues. Structurally, these connecting residues fold into an α-helical domain.[7]
C motif
Directly following the Walker B motif, is the signature motif.[7]
Switch motif
The switch motif has been found to be located at the end of the β4-strand in ATP-binding proteins.[7]
Function
Each ATP binding motif has a different role to play whether it is directly involved with the binding of ATP or helping with the construction of the ATP-binding cassette (ABC) transporter.[6] The ATP molecule binds to the connecting point of each subunit of the dimer, indicating that ATP is in close proximity to both subunits during catalysis. The two binding motifs that ATP directly interacts with is the residues from the Walker A motif, located on one of the subunits, and the residues from the C binding motif, located on the other subunit. The Walker A binding motif has a lysine side chain, which is essential for the binding of ATP. The lysine residue forms hydrogen bonds with the oxygen atoms of two phosphate groups within ATP, therefore creating proximity and orientation of ATP in the binding site.[9][7]
In order for the Walker A motif to bind to ATP, the ATP molecule must be in the binding site. The signature motif acts as a signal to the Walker A motif, letting the Walker A know when the ATP molecule has bound to the binding site. The signature motif does this by allowing its residues to extend from the subunit they are located into the other subunit where the Walker A motif is. It is necessary that ATP binds to both nucleotide binding domains in order to complete the catalytically active structure.[9]
The Walker B motif contains the amino acid glutamate within the short sequence. Glutamate can be used to perform a nucleophilic attack on the ATP molecule.[6]
Found in the switch binding motif is a histidine residue. The function of the histidine is to influence the reaction catalytically by contacting the residues across the dimer interface, including the Walker A motif and the Walker B motif. It is the histidine residue that forms the tight coupling between the binding of the ATP molecule and the dimer.[6][9]
Following the hydrolysis of ADP, a conformational change must occur to separate the ATP-binding cassette. This separation is driven by an electrostatic repulsion by the ADP product that is bound the Walker A motif and the inorganic phosphate product is bound to the C motif.[10]
References
- ↑ Liu, Jinfeng; Rost, Burkhard (2003-02-01). "Domains, motifs and clusters in the protein universe". Current Opinion in Chemical Biology 7 (1): 5–11. doi:10.1016/s1367-5931(02)00003-0. ISSN 1367-5931. PMID 12547420.
- ↑ Chauhan, Jagat S.; Mishra, Nitish K.; Raghava, Gajendra P. S. (2009-12-19). "Identification of ATP binding residues of a protein from its primary sequence". BMC Bioinformatics 10: 434. doi:10.1186/1471-2105-10-434. ISSN 1471-2105. PMID 20021687.
- ↑ 3.0 3.1 G., Voet, Judith; W., Pratt, Charlotte (29 February 2016). Fundamentals of biochemistry : life at the molecular level. John Wiley & Sons. ISBN 9781118918432. OCLC 910538334.
- ↑ Chen, De-Hua; Chang, Andrew Ying-Fei; Liao, Ben-Yang; Yeang, Chen-Hsiang (2017-03-25). "Functional characterization of motif sequences under purifying selection". Nucleic Acids Research 41 (4): 2105–2120. doi:10.1093/nar/gks1456. ISSN 0305-1048. PMID 23303791.
- ↑ Rees, Douglas C.; Johnson, Eric; Lewinson, Oded (2017-03-25). "ABC transporters: The power to change". Nature Reviews Molecular Cell Biology 10 (3): 218–227. doi:10.1038/nrm2646. ISSN 1471-0072. PMID 19234479.
- ↑ 6.0 6.1 6.2 6.3 Hollenstein, Kaspar; Dawson, Roger J. P.; Locher, Kaspar P. (2007-08-01). "Structure and mechanism of ABC transporter proteins". Current Opinion in Structural Biology 17 (4): 412–418. doi:10.1016/j.sbi.2007.07.003. ISSN 0959-440X. PMID 17723295.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 Schneider, Erwin; Hunke, Sabine (1998-04-01). "ATP-binding-cassette (ABC) transport systems: Functional and structural aspects of the ATP-hydrolyzing subunits/domains" (in en). FEMS Microbiology Reviews 22 (1): 1–20. doi:10.1111/j.1574-6976.1998.tb00358.x. ISSN 0168-6445. PMID 9640644.
- ↑ Kumar, Antresh; Shukla, Suneet; Mandal, Ajeet; Shukla, Sudhanshu; Ambudkar, Suresh V.; Prasad, Rajendra (2017-03-07). "Divergent Signature motifs of Nucleotide Binding Domains of ABC multidrug transporter, CaCdr1p of pathogenic Candida albicans, are functionally asymmetric and non interchangeable". Biochimica et Biophysica Acta (BBA) - Biomembranes 1798 (9): 1757–1766. doi:10.1016/j.bbamem.2010.05.017. ISSN 0006-3002. PMID 20546701.
- ↑ 9.0 9.1 9.2 9.3 9.4 Davidson, Amy L.; Dassa, Elie; Orelle, Cedric; Chen, Jue (2008-06-01). "Structure, Function, and Evolution of Bacterial ATP-Binding Cassette Systems" (in en). Microbiology and Molecular Biology Reviews 72 (2): 317–364. doi:10.1128/MMBR.00031-07. ISSN 1092-2172. PMID 18535149.
- ↑ Smith, Paul C.; Karpowich, Nathan; Millen, Linda; Moody, Jonathan E.; Rosen, Jane; Thomas, Philip J.; Hunt, John F. (2017-03-25). "ATP Binding to the Motor Domain from an ABC Transporter Drives Formation of a Nucleotide Sandwich Dimer". Molecular Cell 10 (1): 139–149. doi:10.1016/S1097-2765(02)00576-2. ISSN 1097-2765. PMID 12150914.
 |