Biology:3D bioprinting
Three dimensional (3D) bioprinting is the utilization of 3D printing–like techniques to combine cells, growth factors, bio-inks, and/or biomaterials to fabricate biomedical parts that imitate natural tissue characteristics, form functional biofilms, and assist in the removal of pollutants. 3D bioprinting has uses in fields such as wastewater treatment, environmental remediation, and corrosion prevention.[1] 3D bioprinting can produce functional biofilms which can assist in a variety of situations.[2] The 3D bioprinted biofilms host functional microorganisms which can facilitate pollutant removal.[2] Generally, 3D bioprinting can utilize a layer-by-layer method to deposit materials known as bio-inks to create tissue-like structures that are later used in various medical and tissue engineering fields.[3][4] 3D bioprinting covers a broad range of bioprinting techniques and biomaterials. Currently, bioprinting can be used to print tissue and organ models to help research drugs and potential treatments.[5] Nonetheless, translation of bioprinted living cellular constructs into clinical application is met with several issues due to the complexity and cell number necessary to create functional organs.[6] However, innovations span from bioprinting of extracellular matrix to mixing cells with hydrogels deposited layer by layer to produce the desired tissue.[7] In addition, 3D bioprinting has begun to incorporate the printing of scaffolds which can be used to regenerate joints and ligaments.[8]
Process
File:Bioprinting-of-3D-Convoluted-Renal-Proximal-Tubules-on-Perfusable-Chips-srep34845-s3.ogv
3D bioprinting generally follows three steps: pre-bioprinting, bioprinting, and post-bioprinting.[9][10]
Pre-bioprinting
Pre-bioprinting is the process of creating a model that the printer will later create and choosing the materials that will be used. One of the first steps is to obtain a biopsy of the organ, to sample cells. Common technologies used for bioprinting are computed tomography (CT) and magnetic resonance imaging (MRI). To print with a layer-by-layer approach, tomographic reconstruction is done on the images. The now-2D images are then sent to the printer to be made. Once the image is created, certain cells are isolated and multiplied.[9] These cells are then mixed with a special liquefied material that provides oxygen and other nutrients to keep them alive. This aggregation of cells does not require a scaffold, and is required for placing in the tubular-like tissue fusion for processes such as extrusion.[11]:165
Bioprinting
In the second step, the liquid mixtures of cells, matrix, and nutrients known as bioinks are placed in a printer cartridge and deposited using the patients' medical scans.[12] When a bioprinted pre-tissue is transferred to an incubator, this cell-based pre-tissue matures into a tissue.
3D bioprinting for fabricating biological constructs typically involves dispensing cells onto a biocompatible scaffold using a successive layer-by-layer approach to generate tissue-like three-dimensional structures.[13] Artificial organs such as livers and kidneys made by 3D bioprinting have been shown to lack crucial elements that affect the body such as working blood vessels, tubules for collecting urine, and the growth of billions of cells required for these organs. Without these components the body has no way to get the essential nutrients and oxygen deep within their interiors.[13] Given that every tissue in the body is naturally composed of different cell types, many technologies for printing these cells vary in their ability to ensure stability and viability of the cells during the manufacturing process. Some of the methods that are used for 3D bioprinting of cells are photolithography, magnetic 3D bioprinting, stereolithography, and direct cell extrusion.[11]:196
Post-bioprinting
The post-bioprinting process is necessary to create a stable structure from the biological material. If this process is not well-maintained, the mechanical integrity and function of the 3D printed object is at risk.[9] To maintain the object, both mechanical and chemical stimulations are needed. These stimulations send signals to the cells to control the remodeling and growth of tissues. In addition, in recent development, bioreactor technologies[14] have allowed the rapid maturation of tissues, vascularization of tissues and the ability to survive transplants.[10]
Bioreactors work in either providing convective nutrient transport, creating microgravity environments, changing the pressure causing solution to flow through the cells, or adding compression for dynamic or static loading. Each type of bioreactor is ideal for different types of tissue, for example compression bioreactors are ideal for cartilage tissue.[11]:198
Bioprinting approach
Researchers in the field have developed approaches to produce living organs that are constructed with the appropriate biological and mechanical properties. 3D bioprinting is based on three main approaches: biomimicry, autonomous self-assembly and mini-tissue building blocks.[15]
Biomimicry
The first approach of bioprinting is called biomimicry. The main goal of this approach is to create fabricated structures that are identical to the natural structure that are found in the tissues and organs in the human body. Biomimicry requires duplication of the shape, framework, and the microenvironment of the organs and tissues.[16] The application of biomimicry in bioprinting involves creating both identical cellular and extracellular parts of organs. For this approach to be successful, the tissues must be replicated on a micro scale. Therefore, it is necessary to understand the microenvironment, the nature of the biological forces in this microenvironment, the precise organization of functional and supporting cell types, solubility factors, and the composition of extracellular matrix.[15]
Autonomous self-assembly
The second approach of bioprinting is autonomous self-assembly. This approach relies on the physical process of embryonic organ development as a model to replicate the tissues of interest.[16] When cells are in their early development, they create their own extracellular matrix building block, the proper cell signaling, and independent arrangement and patterning to provide the required biological functions and micro-architecture.[15] Autonomous self-assembly demands specific information about the developmental techniques of the tissues and organs of the embryo.[16] There is a "scaffold-free" model that uses self-assembling spheroids that subjects to fusion and cell arrangement to resemble evolving tissues. Autonomous self-assembly depends on the cell as the fundamental driver of histogenesis, guiding the building blocks, structural and functional properties of these tissues. It demands a deeper understanding of how embryonic tissues mechanisms develop as well as the microenvironment surrounded to create the bioprinted tissues.[15]
Mini-tissue
The third approach of bioprinting is a combination of both the biomimicry and self-assembly approaches, called mini tissues. Organs and tissues are built from very small functional components. The mini-tissue approach takes these small pieces and arrange them into larger framework.[16][15]
Classification of bioprinters
Akin to ordinary ink printers, bioprinters have three major components to them. These are the hardware used, the type of bio-ink, and the material it is printed on (biomaterials).[9] Bio-ink is a material made from living cells that behaves much like a liquid, allowing people to 'print' it in order to create the desired shape. To make bio-ink, scientists create a slurry of cells that can be loaded into a cartridge and inserted into a specially designed printer, along with another cartridge containing a gel known as bio-paper."[17] In bioprinting, there are three major types of printers that have been used. These are inkjet, laser-assisted, and extrusion printers. Inkjet printers are mainly used in bioprinting for fast and large-scale products. One type of inkjet printer, called drop-on-demand inkjet printer, prints materials in exact amounts, minimizing cost and waste.[18] Printers that utilize lasers provide high-resolution printing; however, these printers are often expensive. Extrusion printers print cells layer-by-layer, just like 3D printing to create 3D constructs. In addition to just cells, extrusion printers may also use hydrogels infused with cells.[9]
Extrusion-based
Extrusion-based printing is a very common technique within the field of 3D printing which entails extruding, or forcing, a continuous stream of melted solid material or viscous liquid through a sort of orifice, often a nozzle or syringe.[19] When it comes to extrusion based bioprinting, there are four main types of extrusion. These are pneumatic driven, piston driven, screw driven and eccentric screw driven (also known as progressing cavity pump). Each extrusion method has their own advantages and disadvantages. Pneumatic extrusion uses pressurized air to force liquid bioink through a depositing agent. Air filters are commonly used to sterilize the air before it is used, to ensure air pushing the bioink is not contaminated.[20] Piston driven extrusion utilizes a piston connected to a guide screw. The linear motion of the piston squeezes material out of the nozzle. Screw driven extrusion uses an auger screw to extrude material using rotational motion.[21] Screw driven devices allow for the use of higher viscosity materials and provide more volumetric control.[19] Eccentric screw driven systems allow for a much more precise deposition of low to high viscosity materials due to the self-sealing chambers in the extruder.[22] Once printed, many materials require a crosslinking step to achieve the desired mechanical properties for the construct, which can be achieved for example with the treatment of chemical agents or photo-crosslinkers.
Direct extrusion is one of the most common extrusion-based bioprinting techniques, wherein the pressurized force directs the bioink to flow out of the nozzle, and directly print the scaffold without any necessary casting.[23] The bioink itself for this approach can be a blend of polymer hydrogels, naturally derived materials such as collagen, and live cells suspended in the solution.[23] In this manner, scaffolds can be cultured post-print and without the need for further treatment for cellular seeding. Some focus in the use of direct printing techniques is based upon the use of coaxial nozzle assemblies, or coaxial extrusion. The coaxial nozzle setup enables the simultaneous extrusion of multiple material bioinks, capable of making multi-layered scaffolds in a single extrusion step.[24] The development of tubular structures has found the layered extrusion achieved via these techniques desirable for the radial variability in material characterization that it can offer, as the coaxial nozzle provides an inner and outer tube for bioink flow.[24] Indirect extrusion techniques for bioprinting rather require the printing of a base material of cell-laden hydrogels, but unlike direct extrusion contains a sacrificial hydrogel that can be trivially removed post-printing through thermal or chemical extraction.[25] The remaining resin solidifies and becomes the desired 3D-printed construct.
Laser-based
Laser-based bioprinting can be split into two major classes: those based on cell transfer technologies or photo-polymerization. In cell transfer laser printing, a laser stimulates the connection between energy-absorbing material (e.g. gold, titanium, etc.) and the bioink. This ‘donor layer’ vaporizes under the laser’s irradiation, forming a bubble from the bioink layer which gets deposited from a jet.[26] Photo-polymerization techniques rather use photoinitiated reactions to solidify the ink, moving the beam path of a laser to induce the formation of a desired construct. Certain laser frequencies paired with photopolymerization reactions can be carried out without damaging cells in the material.
Fixed deposition modelling
In this form of printing, plastic residues are melted down and individual layered in sections to create a desired shape. Nylon and PVA are examples of biomaterials utilized in this method. This technique is most often used to design prototypes for prosthetics and cartilage construction.[27]
Inkjet
Another form of bioprinting involves an inkjet printer, which is primarily utilized in biomedical settings. This method prints detailed proteins and nucleic acids.[27] Hydrogels are commonly selected as the bioink. Cells can be printed on to a selected surface media to proliferate and ultimately differentiate. A drawback of this printing method is the ability of the bioinks such as hydrogels to clog the printing nozzle, due to their high viscosity.[27] Ideal inkjet bioprinting involves using a low polymer viscosity (ideally below 10 centipoise), low cell density (<10 million cells/mL), and low structural heights (<10 million cells/mL).[28]
Additional printing methods
There are several other bioprinting techniques which are less commonly used. Droplet-based bioprinting is a technique in which the bioink blend of cells and/or hydrogels are placed in droplets in precise positions. Most common amongst this approach are thermal and piezoelectric-drop-on-demand techniques.[29] This method of bioprinting is often used experimentally with lung and ovarian cancer models.[30] Thermal technologies use short duration signals to heat the bioink, inducing the formation of small bubbles which are ejected. Piezoelectric bioprinting has short duration current applied to a piezoelectric actuator, which induces a mechanical vibration capable of ejecting a small globule of bioink through the nozzle. A significant aspect of the study of droplet-based approaches to bioprinting is accounting for mechanical and thermal stress cells within the bioink experience near the nozzle-tip as they are extruded.
| Method of bioprinting | Mode of printing | Advantages |
|---|---|---|
| Direct printing | Extrusion-based | Simple execution, no casting |
| Coaxial extrusion | Extrusion-based | Single step formation of multi-layered constructs |
| Indirect | Extrusion-based | Requires a removeable 'sacrificial material' to support structural formation |
| Laser | Laser-based | No shear stress upon cells suspended in ink |
| Droplet | Droplet-based | Precise control over flow & formation of scaffold |
Significance of bioink selection
Bioinks are essential components of the bioprinting process. They are composed of living cells and enzymatic supplements to nurture an environment that supports the biological needs of the printed tissue.[31] The environment created by the bioink allows for the cell to attach, grow, and differentiate into its adult form.[31] Cell-encapsualting hydrogels are utilized in extrusion based bioprinting methods, while gelatin MethacryloylGelatin methacrylon (GelMA) and acellular comprised bioinks are most often used in tissue engineering techniques that require cross-linkage and precise structural integrity.[31] It is essential for bioinks to help replicate the external cellular matrix environment that the cell would naturally occur in.
Applications
Transplantation
3D bioprinting can be used to reconstruct tissue from various regions of the body. The precursor to the adoption of 3D printing in healthcare was a series of trials conducted by researchers at Boston Children's Hospital. The team built replacement urinary bladders by hand for seven patients by constructing scaffolds, then layering the scaffolds with cells from the patients and allowing them to grow. The trials were a success as the patients remained in good health 7 years after implantation, which led a research fellow named Anthony Atala, MD, to search or ways to automate the process.[32] Patients with end-stage bladder disease can now be treated by using bio-engineered bladder tissues to rebuild the damaged organ.[33] This technology can also potentially be applied to bone, skin, cartilage and muscle tissue.[34] Though one long-term goal of 3D bioprinting technology is to reconstruct an entire organ as well as minimize the problem of the lack of organs for transplantation.[35] There has been little success in bioprinting of fully functional organs e.g. liver, skin, meniscus or pancreas.[36][37][38] Unlike implantable stents, organs have complex shapes and are significantly harder to bioprint. A bioprinted heart, for example, must not only meet structural requirements, but also vascularization, mechanical load, and electrical signal propagation requirements.[39]
In 2022, first success of a clinical trial for a 3D bioprinted transplant that is made from the patient's own cells, an external ear to treat microtia,[40] was reported.[41]
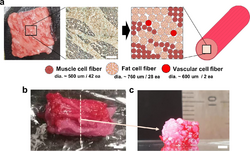
Cultured meat
Bioprinting can also be used for cultured meat. In 2021, a steak-like cultured meat, composed of three types of bovine cell fibers was produced. The Wagyu-like beef has a structure similar to original meat.[42][43] This technology provides an alternative to natural meat harvesting methods if the livestock industry is plagued by disease. In addition, it provides a possible solution to reducing the environmental impact of the livestock industry.
Impact
3D bioprinting contributes to significant advances in the medical field of tissue engineering by allowing for research to be done on innovative materials called biomaterials. Some of the most notable bioengineered substances are usually stronger than the average bodily materials, including soft tissue and bone. These constituents can act as future substitutes, even improvements, for the original body materials. In addition, the Defense Threat Reduction Agency aims to print mini organs such as hearts, livers, and lungs as the potential to test new drugs more accurately and perhaps eliminate the need for testing in animals.[12]
Biofilms
The bioprinting of biofilms utilizes the same methods as other bioprinting. Oftentimes, the biofilm begins with an extrusion of a polysaccharide to provide structure for biofilm growth. An example of one of these polysaccharides is alginate. The alginate structure can have microbes embedded within the structure.[44] Hydrogels can also be used to assist in the formation of functional biofilms.[1] Biofilms are difficult to analyze in a laboratory setting due to the complex structure and the time it takes for a functional biofilm to form. 3D bioprinting biofilms allows us to skip certain processes and makes it easier to analyze functional biofilms.[44] Thickness of the biofilm being printed with change the functionality due to nutrient and oxygen diffusion. Thicker 3D printed biofilms will naturally select for anaerobes for example.[45]
Environmental remediation
Biofilms are capable of remediation in the natural environment which suggests there is potential in regards to the use of 3D bioprinted biofilm use in environmental remediation.[46] Microbes are able to degrade a large range of chemicals and metals and providing a structure for these microbes to flourish such as in biofilm structures is beneficial.[47] Artificial biofilms protect the microbes from the dangers of the environment while promoting signaling and overall microbial interactions.[48] 3D bioprinting allows functional microorganisms to be placed in a structure that provides mechanical stability and protects them from environmental conditions.[2] The larger contact area provided by 3D printed structures compared to normal environmental structures provides more efficient removal of pollutants.[2]
Future uses
Bioprinting also has possible uses in the future in assisting in wastewater treatment and in corrosion control.[44] When humans come in contact with environmental biofilms, it is possible for infections and long-term health hazards to occur.[48] Antibiotic penetration and expansion within a biofilm is an area of research which can benefit from bioprinting techniques, to further explore the effect of environmental biofilms on human health.[2] Biofilm printing requires further research due to limited published data and complex protocols.
See also
- 3D printing § Bio-printing
- Biofabrication
- Cultured meat
- Ethics of bioprinting
- Regenerative medicine
- Bioinks
References
- ↑ 1.0 1.1 Lehner, Benjamin A. E.; Schmieden, Dominik T.; Meyer, Anne S. (2017-03-01). "A Straightforward Approach for 3D Bacterial Printing". ACS Synthetic Biology 6 (7): 1124–1130. doi:10.1021/acssynbio.6b00395. ISSN 2161-5063. PMID 28225616. PMC 5525104. http://dx.doi.org/10.1021/acssynbio.6b00395.
- ↑ 2.0 2.1 2.2 2.3 2.4 Zhao, Tianyang; Liu, Yinuo; Wu, Yichen; Zhao, Minghao; Zhao, Yingxin (2023-12-01). "Controllable and biocompatible 3D bioprinting technology for microorganisms: Fundamental, environmental applications and challenges". Biotechnology Advances 69: 108243. doi:10.1016/j.biotechadv.2023.108243. ISSN 0734-9750. PMID 37647974. https://www.sciencedirect.com/science/article/pii/S0734975023001507.
- ↑ "Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery". European Journal of Cardio-Thoracic Surgery 58 (3): 500–510. 2020. doi:10.1093/ejcts/ezaa093. PMID 32391914.
- ↑ "Advanced Bioinks for 3D Printing: A Materials Science Perspective". Annals of Biomedical Engineering 44 (6): 2090–2102. 2016. doi:10.1007/s10439-016-1638-y. PMID 27184494.
- ↑ "Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels". Science Advances 1 (9): e1500758. October 2015. doi:10.1126/sciadv.1500758. PMID 26601312. Bibcode: 2015SciA....1E0758H.
- ↑ Murphy, Sean V.; De Coppi, Paolo; Atala, Anthony (April 2020). "Opportunities and challenges of translational 3D bioprinting" (in en). Nature Biomedical Engineering 4 (4): 370–380. doi:10.1038/s41551-019-0471-7. ISSN 2157-846X. PMID 31695178. https://www.nature.com/articles/s41551-019-0471-7.
- ↑ "Printability, durability, contractility and vascular network formation in 3D bioprinted cardiac endothelial cells using alginate–gelatin hydrogels". Frontiers in Bioengineering and Biotechnology 9: 110. 2021. doi:10.3389/fbioe.2021.636257. PMID 33748085.
- ↑ "Bone and Joint Diseases in Present and Future". Fukuoka Igaku Zasshi = Hukuoka Acta Medica 108 (1): 1–7. January 2017. PMID 29226660.
- ↑ 9.0 9.1 9.2 9.3 9.4 "Printing Technologies for Medical Applications". Trends in Molecular Medicine 22 (3): 254–265. March 2016. doi:10.1016/j.molmed.2016.01.003. PMID 26856235.
- ↑ 10.0 10.1 "Bioprinting scale-up tissue and organ constructs for transplantation". Trends in Biotechnology 33 (7): 395–400. July 2015. doi:10.1016/j.tibtech.2015.04.005. PMID 25978871.
- ↑ 11.0 11.1 11.2 Bioprinting: Principles and Applications. Singapore: World Scientific Publishing Co. 2015. ISBN 9789814612104. https://books.google.com/books?id=nhK3CgAAQBAJ.
- ↑ 12.0 12.1 "How 3D Printing Could End The Deadly Shortage Of Donor Organs". Huffpost Science. TheHuffingtonPost.com, Inc. 1 March 2015. http://www.huffingtonpost.com/2015/03/01/3d-printed-organs-regenerative-medicine_n_6698606.html.
- ↑ 13.0 13.1 "A sweet solution for replacing organs". Scientific American 308 (4): 54–55. 2013. doi:10.1038/scientificamerican0413-54. Bibcode: 2013SciAm.308d..54H. https://bioengineering.rice.edu/uploadedFiles/Bioengineering/The_Big_Picture/scientificamerican0413-54.pdf. Retrieved 17 February 2016.
- ↑ "Advances in medical polymer technology towards the panacea of complex 3D tissue and organ manufacture". American Journal of Surgery 217 (4): 807–808. April 2019. doi:10.1016/j.amjsurg.2018.05.012. PMID 29803500.
- ↑ 15.0 15.1 15.2 15.3 15.4 "3D bioprinting of tissues and organs". Nature Biotechnology 32 (8): 773–85. August 2014. doi:10.1038/nbt.2958. PMID 25093879.
- ↑ 16.0 16.1 16.2 16.3 "Bio-printing: 3D printing comes to life". Manufacturing Engineering Suppl.. 2015. ProQuest 1678889578.
- ↑ Basic Dental Materials. JP Medical Ltd. 2015. ISBN 9789352500482. https://books.google.com/books?id=7cAqCwAAQBAJ&pg=PA417.
- ↑ "3D Printing Technology At The Service Of Health". healthyeve. http://www.healthyeve.com/3d-printing-technology-service-health/.
- ↑ 19.0 19.1 "Towards preserving post-printing cell viability and improving the resolution: Past, present, and future of 3D bioprinting theory". Bioprinting 11: e00034. 2018. doi:10.1016/j.bprint.2018.e00034. ISSN 2405-8866. http://home.ku.edu.tr/~mmuradoglu/LepowskyEtAlBioprinting2018.pdf.
- ↑ "Development of 3D bioprinting: From printing methods to biomedical applications". Asian Journal of Pharmaceutical Sciences 15 (5): 529–557. September 2020. doi:10.1016/j.ajps.2019.11.003. PMID 33193859.
- ↑ "3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances". Bioactive Materials 3 (2): 144–156. June 2018. doi:10.1016/j.bioactmat.2017.11.008. PMID 29744452.
- ↑ Fisch, Philipp; Holub, Martin; Zenobi-Wong, Marcy (2021-01-01). "Improved accuracy and precision of bioprinting through progressive cavity pump-controlled extrusion". Biofabrication 13 (1): 015012. doi:10.1088/1758-5090/abc39b. ISSN 1758-5082. PMID 33086207. https://iopscience.iop.org/article/10.1088/1758-5090/abc39b.
- ↑ 23.0 23.1 Datta, Pallab; Ayan, Bugra; Ozbolat, Ibrahim T. (March 2017). "Bioprinting for vascular and vascularized tissue biofabrication". Acta Biomaterialia 51: 1–20. doi:10.1016/j.actbio.2017.01.035. ISSN 1742-7061. PMID 28087487.
- ↑ 24.0 24.1 Gupta, Prerak; Mandal, Biman B. (2021-06-12). "Tissue-Engineered Vascular Grafts: Emerging Trends and Technologies". Advanced Functional Materials 31 (33): 2100027. doi:10.1002/adfm.202100027. ISSN 1616-301X. http://dx.doi.org/10.1002/adfm.202100027.
- ↑ Hinton, Thomas J.; Jallerat, Quentin; Palchesko, Rachelle N.; Park, Joon Hyung; Grodzicki, Martin S.; Shue, Hao-Jan; Ramadan, Mohamed H.; Hudson, Andrew R. et al. (2015-10-30). "Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels" (in en). Science Advances 1 (9): e1500758. doi:10.1126/sciadv.1500758. ISSN 2375-2548. PMID 26601312. Bibcode: 2015SciA....1E0758H.
- ↑ Devillard, Raphaël; Pagès, Emeline; Correa, Manuela Medina; Kériquel, Virginie; Rémy, Murielle; Kalisky, Jérôme; Ali, Muhammad; Guillotin, Bertrand et al. (2014), "Cell Patterning by Laser-Assisted Bioprinting" (in en), Micropatterning in Cell Biology Part A, Methods in Cell Biology, 119, Elsevier, pp. 159–174, doi:10.1016/b978-0-12-416742-1.00009-3, ISBN 978-0-12-416742-1, PMID 24439284, https://linkinghub.elsevier.com/retrieve/pii/B9780124167421000093, retrieved 2021-10-27
- ↑ 27.0 27.1 27.2 Sachdev, Ankita; Acharya, Sourya; Gadodia, Tejas; Shukla, Samarth; J, Harshita; Akre, Chinmay; Khare, Mansi; Huse, Shreyash (2022). "A Review on Techniques and Biomaterials Used in 3D Bioprinting". Cureus 14 (8): e28463. doi:10.7759/cureus.28463. ISSN 2168-8184. PMID 36176831.
- ↑ Hansen, Christopher J.; Saksena, Rajat; Kolesky, David B.; Vericella, John J.; Kranz, Stephen J.; Muldowney, Gregory P.; Christensen, Kenneth T.; Lewis, Jennifer A. (2013-01-04). "High-throughput printing via microvascular multinozzle arrays". Advanced Materials (Deerfield Beach, Fla.) 25 (1): 96–102. doi:10.1002/adma.201203321. ISSN 1521-4095. PMID 23109104. Bibcode: 2013AdM....25...96H. https://pubmed.ncbi.nlm.nih.gov/23109104/.
- ↑ Munaz, Ahmed; Vadivelu, Raja K.; St. John, James; Barton, Matthew; Kamble, Harshad; Nguyen, Nam-Trung (March 2016). "Three-dimensional printing of biological matters". Journal of Science: Advanced Materials and Devices 1 (1): 1–17. doi:10.1016/j.jsamd.2016.04.001. ISSN 2468-2179.
- ↑ Maharjan, Dr. S.; Bonilla, Ms.D.; Zhang, Prof. Y. S. (2019). "Three-Dimensional Bioprinting for Tissue and Disease Modeling". https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/materials-science-and-engineering/3d-bioprinting/3d-bioprinting-tissue0.
- ↑ 31.0 31.1 31.2 "3D Bioprinting: Bioink Selection Guide". 2023. https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/3d-cell-culture/3d-bioprinting-bioinks.
- ↑ Whitaker, Matthew (July 2014). "The history of 3D printing in healthcare" (in en). The Bulletin of the Royal College of Surgeons of England 96 (7): 228–229. doi:10.1308/147363514X13990346756481. ISSN 1473-6357.
- ↑ "Tissue-engineered autologous bladders for patients needing cystoplasty". Lancet 367 (9518): 1241–6. April 2006. doi:10.1016/S0140-6736(06)68438-9. PMID 16631879.
- ↑ "3D bioprinting and its in vivo applications". Journal of Biomedical Materials Research Part B: Applied Biomaterials 106 (1): 444–459. January 2018. doi:10.1002/jbm.b.33826. PMID 28106947.
- ↑ Shinkar, Kalyani; Rhode, Kawal (2022-08-01). "Could 3D extrusion bioprinting serve to be a real alternative to organ transplantation in the future?". Annals of 3D Printed Medicine 7: 100066. doi:10.1016/j.stlm.2022.100066. ISSN 2666-9641.
- ↑ "90-OR: 3D Bioprinting of a Bionic Pancreas with a Vascular System—Results of Transplantation in Large Animals". https://diabetesjournals.org/diabetes/article/72/Supplement_1/90-OR/149847/90-OR-3D-Bioprinting-of-a-Bionic-Pancreas-with-a.
- ↑ "Implementations of 3D printing in ophthalmology". Graefe's Archive for Clinical and Experimental Ophthalmology 257 (9): 1815–1822. September 2019. doi:10.1007/s00417-019-04312-3. PMID 30993457.
- ↑ Klak, Marta; Bryniarski, Tomasz; Kowalska, Patrycja; Gomolka, Magdalena; Tymicki, Grzegorz; Kosowska, Katarzyna; Cywoniuk, Piotr; Dobrzanski, Tomasz et al. (2020-06-30). "Novel Strategies in Artificial Organ Development: What Is the Future of Medicine?". Micromachines 11 (7): 646. doi:10.3390/mi11070646. ISSN 2072-666X. PMID 32629779.
- ↑ "3D bioprinting for cardiovascular regeneration and pharmacology". Advanced Drug Delivery Reviews 132: 252–269. July 2018. doi:10.1016/j.addr.2018.07.014. PMID 30053441.
- ↑ "A Multicenter, Single Arm, Prospective, Open-Label, Staged Study of the Safety and Efficacy of the AuriNovo Construct for Auricular Reconstruction in Subjects With Unilateral Microtia". clinicaltrials.gov. 15 October 2021. https://clinicaltrials.gov/ct2/show/NCT04399239.
- ↑ Rabin, Roni Caryn (2 June 2022). "Doctors Transplant Ear of Human Cells, Made by 3-D Printer". https://www.nytimes.com/2022/06/02/health/ear-transplant-3d-printer.html.
- ↑ "Japanese scientists produce first 3D-bioprinted, marbled Wagyu beef". New Atlas. 25 August 2021. https://newatlas.com/science/world-first-lab-grown-wagyu-beef-japan/.
- ↑ "Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting". Nature Communications 12 (1): 5059. August 2021. doi:10.1038/s41467-021-25236-9. PMID 34429413. Bibcode: 2021NatCo..12.5059K.
- ↑ 44.0 44.1 44.2 Balasubramanian, Srikkanth; Yu, Kui; Vasquez Cardenas, Diana; Aubin-Tam, Marie-Eve; Meyer, Anne S. (2021). "Emergent Biological Endurance Depends on Extracellular Matrix Composition of Three-Dimensionally Printed Escherichia coli Biofilms". ACS Synthetic Biology 10 (11): 2997–3008. doi:10.1021/acssynbio.1c00290.s002. PMID 34652130. PMC 8609572. http://dx.doi.org/10.1021/acssynbio.1c00290.s002. Retrieved 2023-09-30.
- ↑ Ning, Evita; Turnbull, Gareth; Clarke, Jon; Picard, Fred; Riches, Philip; Vendrell, Marc; Graham, Duncan; Wark, Alastair W. et al. (2019-09-13). "3D bioprinting of mature bacterial biofilms for antimicrobial resistance drug testing". Biofabrication 11 (4): 045018. doi:10.1088/1758-5090/ab37a0. ISSN 1758-5090. PMID 31370051. Bibcode: 2019BioFa..11d5018N.
- ↑ Mishra, Sandhya; Huang, Yaohua; Li, Jiayi; Wu, Xiaozhen; Zhou, Zhe; Lei, Qiqi; Bhatt, Pankaj; Chen, Shaohua (2022). "Biofilm-mediated bioremediation is a powerful tool for the removal of environmental pollutants". Chemosphere 294: 133609. doi:10.1016/j.chemosphere.2022.133609. ISSN 0045-6535. PMID 35051518. Bibcode: 2022Chmsp.294m3609M. http://dx.doi.org/10.1016/j.chemosphere.2022.133609.
- ↑ Sonawane, Jayesh M.; Rai, Ashutosh Kumar; Sharma, Minaxi; Tripathi, Manikant; Prasad, Ram (2022). "Microbial biofilms: Recent advances and progress in environmental bioremediation". Science of the Total Environment 824: 153843. doi:10.1016/j.scitotenv.2022.153843. ISSN 0048-9697. PMID 35176385. Bibcode: 2022ScTEn.824o3843S. http://dx.doi.org/10.1016/j.scitotenv.2022.153843.
- ↑ 48.0 48.1 Liu, Yifei; Xia, Xiudong; Liu, Zhen; Dong, Mingsheng (2022-12-22). "The Next Frontier of 3D Bioprinting: Bioactive Materials Functionalized by Bacteria". Small 19 (10): e2205949. doi:10.1002/smll.202205949. ISSN 1613-6810. PMID 36549677. http://dx.doi.org/10.1002/smll.202205949.
Further reading
- "To Bioprint or Not to Bioprint". North Carolina Journal of Law and Technology 17: 123–78. 2015. http://ncjolt.org/to-bioprint-or-not-to-bioprint-2/. Retrieved 2019-01-12.
- "Patenting Bioprinting". Harvard Journal of Law and Technology Digest 29. May 7, 2015.
- Stem Cell Biology and Tissue Engineering in Dental Sciences. Elsevier, 2014. 2014-11-27. ISBN 9780123971579. https://books.google.com/books?id=22ipoAEACAAJ.
 |