Chemistry:Nickel bis(dimethyldithiocarbamate)
From HandWiki
Revision as of 14:02, 17 July 2022 by imported>QCDvac (add)
| |
| |
| Names | |
|---|---|
| Other names
Sankel, ethyl niclate; nickel dimethyldithiocarbamate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H12N2NiS4 | |
| Molar mass | 299.11 g·mol−1 |
| Appearance | black solid |
| insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
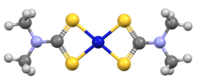
Nickel bis(dimethyldithiocarbamate) is the coordination complex on nickel and dimethyldithiocarbamate, with the formula Ni(S2CNMe2)2 (Me = methyl). It is the prototype for a large number of bis(dialkhyldithiocarbamate)s of nickel(II), which feature diverse organic substituents, all of which have similar structures. Nickel bis(dimethyldithiocarbamate) has been marketed as a fungicide and related complexes are used as stabilizers in polymers.[1]
Preparation and structure
The compound precipitates as a black solid upon combining aqueous solutions of salts of nickel(II) and dimethyldithiocarbamate. In terms of structure and bonding the nickel is square planar, and the complex is diamagnetic.[2]
See also
- Zinc dimethyldithiocarbamate - a related compound where nickel has been replaced with zinc,
- Iron tris(dimethyldithiocarbamate) - a related compound with three dithiocarbamate ligands coordinated to iron.
References
- ↑ Rüdiger Schubart (2000). "Dithiocarbamic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_001.
- ↑ D. Coucouvanis. "The Chemistry of the Dithioacid and 1,1-Dithiolate Complexes". Progress in Inorganic Chemistry 11: 233–371. doi:10.1002/9780470166123.ch4.
 |

