Chemistry:2,2-Dimethoxypropane
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,2-Dimethoxypropane | |||
| Other names
acetone dimethyl acetal
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C5H12O2 | |||
| Molar mass | 104.15 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.85 g/cm3 | ||
| Melting point | −47 °C (−53 °F; 226 K) | ||
| Boiling point | 83 °C (181 °F; 356 K) | ||
| 15 g/L (20 °C) | |||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H225, H315, H319, H335 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P403+235, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
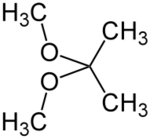
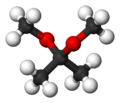
2,2-Dimethoxypropane (DMP) is an organic compound with the formula (CH3)2C(OCH3)2. A colorless liquid, it is the product of the condensation of acetone and methanol. DMP is used as a water scavenger in water-sensitive reactions. Upon acid-catalyzed reaction, DMP reacts quantitatively with water to form acetone and methanol.[2] This property can be used to accurately determine the amount of water in a sample, alternatively to the Karl Fischer method.[3]
DMP is specifically used to prepare acetonides:[4][5]
- RCHOHCHOHCH2 + (MeO)2CMe2 → RCHCHCH2O2CMe2 + 2 MeOH
Dimethoxypropane is an intermediate for the synthesis of 2-methoxypropene.
In histology, DMP is used for the dehydration of animal tissue.[6]
References
- ↑ 2,2-Dimethoxypropane at Sigma-Aldrich
- ↑ Critchfield, F. E.; Bishop, E. T. (1961). "Water determination by reaction with 2, 2-dimethoxypropane". Anal. Chem. 33 (8): 1034. doi:10.1021/ac60176a051.
- ↑ Martin, J. H.; Knevel, A. M. (1965). "Gas chromatographic method of moisture determination". J. Pharm. Sci. 54 (10): 1464–7. doi:10.1002/jps.2600541013. PMID 5883217.
- ↑ Christopher R. Schmid; Jerry D. Bryant (1995). "D-(R)-Glyceraldehyde Acetonide". Org. Synth. 72: 6. doi:10.15227/orgsyn.072.0006.
- ↑ Christian Hubschwerlen; Jean-luc Specklin; J. Higelin (1995). "L-(S)-glyceraldehyde Acetonide". Org. Synth. 72: 1. doi:10.15227/orgsyn.072.0001.
- ↑ Poul Prentø (1978). "Rapid dehydration--clearing with 2,2-dimethoxypropane for paraffin embedding". J. Histochem. Cytochem. 26 (10): 865–867. doi:10.1177/26.10.363931. PMID 363931.
External links
 |